Role of gamma interferon in chemokine expression in the ileum of mice and in a murine intestinal epithelial cell line after Cryptosporidium parvum infection
- PMID: 11895975
- PMCID: PMC127832
- DOI: 10.1128/IAI.70.4.2090-2099.2002
Role of gamma interferon in chemokine expression in the ileum of mice and in a murine intestinal epithelial cell line after Cryptosporidium parvum infection
Abstract
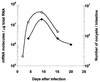
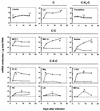
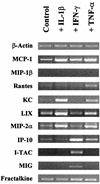
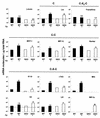
Cryptosporidium parvum is a protozoan parasite that infects intestinal epithelial cells and induces inflammation of the intestine. To better understand the inflammatory process occurring during cryptosporidiosis, we investigated in this study the kinetics of chemokine expression in the mucosa of mice by quantitative reverse transcription-PCR. Our results demonstrate that among the chemokine mRNAs studied, gamma interferon (IFN-gamma)-inducible protein 10 (IP-10), monokine induced by IFN-gamma (MIG), i-TAC, lymphotactin, macrophage inflammatory protein 1 beta (MIP-1 beta), and RANTES mRNAs were strongly up-regulated in infected neonate mice, which correlated with the immunofluorescence staining results showing T-cell and macrophage infiltration in the mucosa. Our in vitro data showed that intestinal epithelial cells infected by C. parvum or stimulated by the proinflammatory cytokines (IFN-gamma, interleukin-1 beta, and tumor necrosis factor alpha) produce a pattern of chemokine secretion similar to that observed in vivo, suggesting that these cells may take part in the initial production of chemokines. In order to identify the chemokines responsible for the recruitment of the inflammatory cells leading to a protective immune response, we compared the patterns of chemokine expression in a healing neonate mouse model and a nonhealing IFN-gamma knockout (GKO) mouse model of cryptosporidiosis. In the absence of IFN-gamma, the chemokine response was altered for IP-10, MIG, i-TAC, RANTES, and MIP-1 beta mRNAs, while the three ELR C-X-C chemokine mRNAs studied (lipopolysaccharide-induced C-X-C chemokine, MIP-2 alpha, and KC mRNAs) were strongly overexpressed. These results are consistent with the neutrophil recruitment observed in the lamina propria of GKO mice at day 9 postinfection but are not consistent with the hypothesis that these cells play an important role in the resolution of the infection. On the contrary, the altered response of chemokines responsible for the recruitment of macrophages and T cells in GKO mice suggests that these two populations may be critical in the development of a protective immune response.
Figures


References
-
- Adjei, A. A., J. T. Jones, and F. J. Enriquez. 2000. Differential intraepithelial lymphocyte phenotypes following Cryptosporidium parvum challenge in susceptible and resistant athymic strains of mice. Parasitol. Int. 49:119-129. - PubMed
-
- Aliberti, J. C., F. S. Machado, J. T. Souto, A. P. Campanelli, M. M. Teixeira, R. T. Gazzinelli, and J. S. Silva. 1999. β-Chemokines enhance parasite uptake and promote nitric oxide-dependent microbiostatic activity in murine inflammatory macrophages infected with Trypanosoma cruzi. Infect. Immun. 67:4819-4826. - PMC - PubMed
-
- Aliberti, J. C., J. T. Souto, A. P. Marino, J. Lannes-Vieira, M. M. Teixeira, J. Farber, R. T. Gazzinelli, and J. S. Silva. 2001. Modulation of chemokine production and inflammatory responses in interferon-gamma- and tumor necrosis factor-R1-deficient mice during Trypanosoma cruzi infection. Am. J. Pathol. 158:1433-1440. - PMC - PubMed
-
- Amichay, D., R. T. Gazzinelli, G. Karupiah, T. R. Moench, A. Sher, and J. M. Farber. 1996. Genes for chemokines MuMig and Crg-2 are induced in protozoan and viral infections in response to IFN-gamma with patterns of tissue expression that suggest nonredundant roles in vivo. J. Immunol. 157:4511-4520. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

