Pseudomonas aeruginosa ExoT acts in vivo as a GTPase-activating protein for RhoA, Rac1, and Cdc42
- PMID: 11895987
- PMCID: PMC127837
- DOI: 10.1128/IAI.70.4.2198-2205.2002
Pseudomonas aeruginosa ExoT acts in vivo as a GTPase-activating protein for RhoA, Rac1, and Cdc42
Abstract
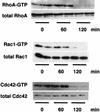
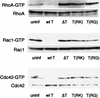
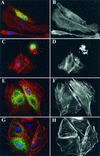
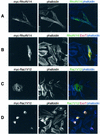
The Pseudomonas aeruginosa protein ExoT is a bacterial GTPase-activating protein (GAP) that has in vitro activity toward Rho, Rac, and Cdc42 GTPases. Expression of ExoT both inhibits the internalization of strain PA103 by macrophages and epithelial cells and is associated with morphological changes (cell rounding and detachment) of infected cells. We find that expression of ExoT leads to the loss of GTP-bound RhoA, Rac1, and Cdc42 in transfected HeLa cells, demonstrating that ExoT has GAP activity in vivo toward all three GTPases. GAP activity is absolutely dependent on the presence of arginine at position 149 but is not affected by whether ExoT is expressed in the absence or presence of other P. aeruginosa type III secreted proteins. We also demonstrate that expression of ExoT in epithelial cells is sufficient to cause stress fiber disassembly by means of ExoT's GAP activity toward RhoA.
Figures

References
-
- Andor, A., K. Trulzsch, M. Essler, A. Roggenkamp, A. Wiedemann, J. Heesemann, and M. Aepfelbacher. 2001. YopE of Yersinia, a GAP for Rho GTPases, selectively modulates Rac-dependent actin structures in endothelial cells. Cell. Microbiol. 3:301-310. - PubMed
-
- Bagrodia, S., S. J. Taylor, K. A. Jordon, L. Van Aelst, and R. A. Cerione. 1998. A novel regulator of p21-activated kinases. J. Biol. Chem. 273:23633-23636. - PubMed
-
- Black, D. B., and J. B. Bliska. 2000. The RhoGAP activity of the Yersinia cytotoxin YopE is required for antiphagocytic function and virulence. Mol. Microbiol. 37:515-527. - PubMed
-
- Bliska, J. B. 2000. Yop effectors of Yersinia spp. and actin rearrangements. Trends Microbiol. 8:205-208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

