Long-term depression in the adult hippocampus in vivo involves activation of extracellular signal-regulated kinase and phosphorylation of Elk-1
- PMID: 11896145
- PMCID: PMC6758273
- DOI: 10.1523/JNEUROSCI.22-06-02054.2002
Long-term depression in the adult hippocampus in vivo involves activation of extracellular signal-regulated kinase and phosphorylation of Elk-1
Abstract
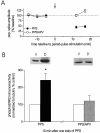
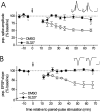
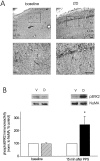
Protein kinase cascades likely play a critical role in the signaling events that underlie synaptic plasticity and memory. The extracellular signal-regulated kinase (ERK) cascade is suited well for such a role because its targets include regulators of gene expression. Here we report that the ERK cascade is recruited during long-term depression (LTD) of synaptic strength in area CA1 of the adult hippocampus in vivo and selectively impacts on phosphorylation of the nuclear transcription factor Elk-1. Using a combination of in vivo electrophysiology, biochemistry, pharmacology, and immunohistochemistry, we found the following: (1) ERK phosphorylation, including phosphorylation of nuclear ERK, and ERK phosphotransferase activity are increased markedly, albeit transiently, after the induction of NMDA receptor-dependent LTD at the commissural input to area CA1 pyramidal cells in the hippocampus of anesthetized adult rats; (2) LTD-inducing paired-pulse stimulation fails to produce lasting LTD in the presence of the ERK kinase inhibitor SL327, which suggests that ERK activation is necessary for the persistence of LTD; and (3) ERK activation during LTD results in increased phosphorylation of Elk-1 but not of the transcription factor cAMP response element-binding protein. Our findings indicate that the ERK cascade transduces signals from the synapse to the nucleus during LTD in hippocampal area CA1 in vivo, as it does during long-term potentiation in area CA1, but that the pattern of coupling of the ERK cascade to transcriptional regulators differs between the two forms of synaptic plasticity.
Figures



References
-
- Abraham WC, Demmer J, Richardson LC, Williams JM, Lawlor PA, Mason SE, Tate WP, Dragunow M. Correlations between early gene induction and the persistence of LTP. Neuroscience. 1993;56:717–727. - PubMed
-
- Alberini CM, Ghirardi M, Huang Y-Y, Nguyen PV, Kandel ER. A molecular switch for the consolidation of long-term memory: cAMP-inducible gene expression. Ann NY Acad Sci. 1995;758:261–286. - PubMed
-
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. - PubMed
-
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. - PubMed
-
- Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
