Dysfunctional light-evoked regulation of cAMP in photoreceptors and abnormal retinal adaptation in mice lacking dopamine D4 receptors
- PMID: 11896146
- PMCID: PMC6758276
- DOI: 10.1523/JNEUROSCI.22-06-02063.2002
Dysfunctional light-evoked regulation of cAMP in photoreceptors and abnormal retinal adaptation in mice lacking dopamine D4 receptors
Abstract
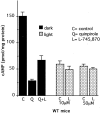
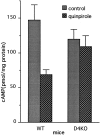
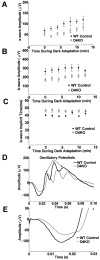
Dopamine is a retinal neuromodulator that has been implicated in many aspects of retinal physiology. Photoreceptor cells express dopamine D4 receptors that regulate cAMP metabolism. To assess the effects of dopamine on photoreceptor physiology, we examined the morphology, electrophysiology, and regulation of cAMP metabolism in mice with targeted disruption of the dopamine D4 receptor gene. Photoreceptor morphology and outer segment disc shedding after light onset were normal in D4 knock-out (D4KO) mice. Quinpirole, a dopamine D2/D3/D4 receptor agonist, decreased cAMP synthesis in retinas of wild-type (WT) mice but not in retinas of D4KO mice. In WT retinas, the photoreceptors of which were functionally isolated by incubation in the presence of exogenous glutamate, light also suppressed cAMP synthesis. Despite the similar inhibition of cAMP synthesis, the effect of light is directly on the photoreceptors and independent of dopamine modulation, because it was unaffected by application of the D4 receptor antagonist l-745,870. Nevertheless, compared with WT retinas, basal cAMP formation was reduced in the photoreceptors of D4KO retinas, and light had no additional inhibitory effect. The results suggest that dopamine, via D4 receptors, normally modulates the cascade that couples light responses to adenylyl cyclase activity in photoreceptor cells, and the absence of this modulation results in dysfunction of the cascade. Dark-adapted electroretinogram (ERG) responses were normal in D4KO mice. However, ERG b-wave responses were greatly suppressed during both light adaptation and early stages of dark adaptation. Thus, the absence of D4 receptors affects adaptation, altering transmission of light responses from photoreceptors to inner retinal neurons. These findings indicate that dopamine D4 receptors normally play a major role in regulating photoreceptor cAMP metabolism and adaptive retinal responses to changing environmental illumination.
Figures





Similar articles
-
Regulation of cAMP by light and dopamine receptors is dysfunctional in photoreceptors of dystrophic retinal degeneration slow(rds) mice.Exp Eye Res. 2001 Aug;73(2):265-72. doi: 10.1006/exer.2001.1037. Exp Eye Res. 2001. PMID: 11446777
-
Prolonged exposure of chicks to light or darkness differentially affects the quinpirole-evoked suppression of serotonin N-acetyltransferase activity in chick retina: an impact on dopamine D4-like receptor.J Pineal Res. 1997 Mar;22(2):59-64. doi: 10.1111/j.1600-079x.1997.tb00304.x. J Pineal Res. 1997. PMID: 9181516
-
Pharmacological and functional characterisation of dopamine D4 receptors in the rat retina.Neuropharmacology. 2003 Jun;44(8):1038-46. doi: 10.1016/s0028-3908(03)00112-6. Neuropharmacology. 2003. PMID: 12763097
-
Dopamine D4-like receptors in vertebrate retina: does the retina offer a model for the D4-receptor analysis?Pol J Pharmacol. 1997 Aug;49(4):201-11. Pol J Pharmacol. 1997. PMID: 9437763 Review.
-
Dopamine receptor localization in the mammalian retina.Mol Neurobiol. 1999 Jun;19(3):181-204. doi: 10.1007/BF02821713. Mol Neurobiol. 1999. PMID: 10495103 Review.
Cited by
-
Dopamine mediates circadian clock regulation of rod and cone input to fish retinal horizontal cells.J Physiol. 2002 Nov 1;544(3):801-16. doi: 10.1113/jphysiol.2002.023671. J Physiol. 2002. PMID: 12411525 Free PMC article.
-
Impaired Light Adaptation of ON-Sustained Ganglion Cells in Early Diabetes Is Attributable to Diminished Response to Dopamine D4 Receptor Activation.Invest Ophthalmol Vis Sci. 2022 Jan 3;63(1):33. doi: 10.1167/iovs.63.1.33. Invest Ophthalmol Vis Sci. 2022. PMID: 35077550 Free PMC article.
-
HCN4-like immunoreactivity in rat retinal ganglion cells.Vis Neurosci. 2008 Jan-Feb;25(1):95-102. doi: 10.1017/S095252380808005X. Vis Neurosci. 2008. PMID: 18282314 Free PMC article.
-
Pre-mRNA Processing Factors and Retinitis Pigmentosa: RNA Splicing and Beyond.Front Cell Dev Biol. 2021 Jul 28;9:700276. doi: 10.3389/fcell.2021.700276. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34395430 Free PMC article. Review.
-
Pineal function: impact of microarray analysis.Mol Cell Endocrinol. 2010 Jan 27;314(2):170-83. doi: 10.1016/j.mce.2009.07.010. Epub 2009 Jul 19. Mol Cell Endocrinol. 2010. PMID: 19622385 Free PMC article. Review.
References
-
- Berson DM, Dunn FA, Takao M. Phototransduction by ganglion cells innervating the circadian pacemaker. Invest Ophthalmol Vis Sci. 2001;42:S113.
-
- Besharse JC, Hollyfield JG. Turnover of mouse photoreceptor outer segments in constant light and darkness. Invest Ophthalmol Vis Sci. 1979;18:1019–1024. - PubMed
-
- Besharse JC, Iuvone PM. Is dopamine a light-adaptive or dark-adaptive modulator in retina? Neurochem Int. 1992;20:193–199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
