Estrogen protects against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA1
- PMID: 11896151
- PMCID: PMC6758282
- DOI: 10.1523/JNEUROSCI.22-06-02115.2002
Estrogen protects against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA1
Abstract
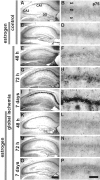
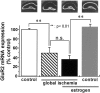
The importance of postmenopausal estrogen replacement therapy in affording protection against the selective and delayed neuronal death associated with cardiac arrest or cardiac surgery in women remains controversial. Here we report that exogenous estrogen at levels that are physiological for hormone replacement in postmenopausal women affords protection against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA1 of male gerbils. Global ischemia induced a marked increase in activated caspase-3 in CA1, evident at 6 hr after ischemia. Global ischemia induced a marked upregulation of the proapoptotic neurotrophin receptor p75(NTR) in CA1, evident at 48 hr. p75(NTR) expression was induced primarily in terminal deoxynucleotidyl transferase-mediated UTP nick-end labeling-positive cells, indicating expression in neurons undergoing apoptosis. Global ischemia also induced a marked downregulation of mRNA encoding the AMPA receptor GluR2 subunit in CA1. Caspase-3, p75(NTR), and GluR2 were not significantly changed in CA3 and dentate gyrus, indicating that the ischemia-induced changes in gene expression were cell specific. Exogenous estrogen attenuated the ischemia-induced increases in activated caspase-3 and blocked the increase in p75(NTR) in post-ischemic CA1 neurons but did not prevent ischemia-induced downregulation of GluR2. These findings demonstrate that long-term estrogen at physiological levels ameliorates ischemia-induced hippocampal injury and indicate that estrogen intervenes at the level of apoptotic signaling cascades to prevent onset of death in neurons otherwise "destined to die."
Figures





Similar articles
-
Late calcium EDTA rescues hippocampal CA1 neurons from global ischemia-induced death.J Neurosci. 2004 Nov 3;24(44):9903-13. doi: 10.1523/JNEUROSCI.1713-04.2004. J Neurosci. 2004. PMID: 15525775 Free PMC article.
-
Hypothermia rescues hippocampal CA1 neurons and attenuates down-regulation of the AMPA receptor GluR2 subunit after forebrain ischemia.Proc Natl Acad Sci U S A. 2003 Mar 4;100(5):2906-10. doi: 10.1073/pnas.2628027100. Epub 2003 Feb 26. Proc Natl Acad Sci U S A. 2003. PMID: 12606709 Free PMC article.
-
Co-induction of p75NTR and p75NTR-associated death executor in neurons after zinc exposure in cortical culture or transient ischemia in the rat.J Neurosci. 2000 Dec 15;20(24):9096-103. doi: 10.1523/JNEUROSCI.20-24-09096.2000. J Neurosci. 2000. PMID: 11124986 Free PMC article.
-
The AMPAR subunit GluR2: still front and center-stage.Brain Res. 2000 Dec 15;886(1-2):190-207. doi: 10.1016/s0006-8993(00)02951-6. Brain Res. 2000. PMID: 11119696 Review.
-
Estradiol rescues neurons from global ischemia-induced cell death: multiple cellular pathways of neuroprotection.Steroids. 2009 Jul;74(7):555-61. doi: 10.1016/j.steroids.2009.01.003. Epub 2009 Jan 20. Steroids. 2009. PMID: 19428444 Free PMC article. Review.
Cited by
-
Vitamin B12 and estradiol benzoate improve memory retrieval through activation of the hippocampal AKT, BDNF, and CREB proteins in a rat model of multiple sclerosis.Iran J Basic Med Sci. 2021 Feb;24(2):256-263. doi: 10.22038/IJBMS.2021.51469.11681. Iran J Basic Med Sci. 2021. PMID: 33953866 Free PMC article.
-
Protective effect of extract of Cordyceps sinensis in middle cerebral artery occlusion-induced focal cerebral ischemia in rats.Behav Brain Funct. 2010 Oct 19;6:61. doi: 10.1186/1744-9081-6-61. Behav Brain Funct. 2010. PMID: 20955613 Free PMC article.
-
Ischemic preconditioning acts upstream of GluR2 down-regulation to afford neuroprotection in the hippocampal CA1.Proc Natl Acad Sci U S A. 2002 Feb 19;99(4):2362-7. doi: 10.1073/pnas.261713299. Epub 2002 Feb 12. Proc Natl Acad Sci U S A. 2002. PMID: 11842229 Free PMC article.
-
LumbarIschemic Optic NeuropathyComplicating Spine Surgery-A Case Report.J Orthop Case Rep. 2019;9(4):58-62. doi: 10.13107/jocr.2019.v09.i04.1480. J Orthop Case Rep. 2019. PMID: 32405490 Free PMC article.
-
Temporal Pattern and Crosstalk of Necroptosis Markers with Autophagy and Apoptosis Associated Proteins in Ischemic Hippocampus.Neurotox Res. 2018 Jul;34(1):79-92. doi: 10.1007/s12640-017-9861-3. Epub 2018 Jan 8. Neurotox Res. 2018. PMID: 29313217
References
-
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165. - PubMed
-
- Bagum MA, Miyamoto O, Toyoshima T, Masada T, Nagahata S, Itano T. The contribution of low affinity NGF receptor (p75NGFR) to delayed neuronal death after ischemia in the gerbil hippocampus. Acta Med Okayama. 2001;55:19–24. - PubMed
-
- Banasiak KJ, Xia Y, Haddad GG. Mechanisms underlying hypoxia-induced neuronal apoptosis. Prog Neurobiol. 2000;62:215–249. - PubMed
-
- Bredesen DE. Neural apoptosis. Ann Neurol. 1995;38:839–851. - PubMed
-
- Chen J, Adachi N, Liu K, Arai T. The effects of 17beta-estradiol on ischemia-induced neuronal damage in the gerbil hippocampus. Neuroscience. 1998a;87:817–822. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
