Localization and regulation of the tissue plasminogen activator-plasmin system in the hippocampus
- PMID: 11896152
- PMCID: PMC6758256
- DOI: 10.1523/JNEUROSCI.22-06-02125.2002
Localization and regulation of the tissue plasminogen activator-plasmin system in the hippocampus
Abstract
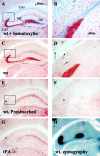
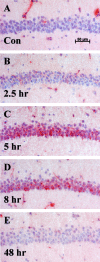
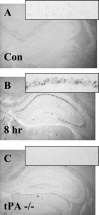
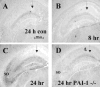
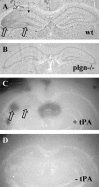
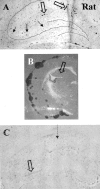
The extracellular protease cascade of tissue plasminogen activator (tPA) and plasminogen has been implicated in neuronal plasticity and degeneration. We show here that unstimulated expression of tPA in the mouse hippocampus is concentrated in the mossy fiber pathway, with little or no expression within the perforant path, the Schaffer collaterals, or neuronal cell bodies. tPA protein is also expressed in vascular endothelial cells throughout the brain parenchyma. Four hours after excitotoxic injury, tPA protein is transiently induced within CA1 pyramidal neurons. The induced CA1 tPA is localized to neurons that survive the injury and is enzymatically active. Within the mossy fiber pathway, injury resulted in decreased tPA protein. In contrast, mossy fiber tPA activity displayed a biphasic character: transient increase at 8 hr, then a decrease by 24 hr after injury. Analysis of plasminogen activator inhibitor-1 (PAI-1) expression showed that PAI-1 antigen is upregulated by 24 hr and could account for the tPA activity downregulation seen at this time point. Plasminogen immunohistochemistry suggested an increase within the mossy fiber pathway after injury. Finally, hippocampal tPA expression among various mammalian species was strikingly different. These results indicate a complex control of tPA protein and enzymatic activity in the hippocampus that may help regulate neuronal plasticity.
Figures


Similar articles
-
The tissue plasminogen activator (tPA)/plasmin extracellular proteolytic system regulates seizure-induced hippocampal mossy fiber outgrowth through a proteoglycan substrate.J Cell Biol. 2000 Mar 20;148(6):1295-304. doi: 10.1083/jcb.148.6.1295. J Cell Biol. 2000. PMID: 10725341 Free PMC article.
-
Phospholipase D1-promoted release of tissue plasminogen activator facilitates neurite outgrowth.J Neurosci. 2005 Feb 16;25(7):1797-805. doi: 10.1523/JNEUROSCI.4850-04.2005. J Neurosci. 2005. PMID: 15716416 Free PMC article.
-
Proteolysis of neuronal cell adhesion molecule by the tissue plasminogen activator-plasmin system after kainate injection in the mouse hippocampus.Neurosci Res. 1999 Jan;33(1):1-8. doi: 10.1016/s0168-0102(98)00105-9. Neurosci Res. 1999. PMID: 10096465
-
Role of annexin II tetramer in plasminogen activation.Trends Cardiovasc Med. 1999 Apr-May;9(3-4):92-102. doi: 10.1016/s1050-1738(99)00012-2. Trends Cardiovasc Med. 1999. PMID: 10578524 Review.
-
[Role of tissue plasminogen activator in the rewarding effect of morphine].Nihon Arukoru Yakubutsu Igakkai Zasshi. 2006 Feb;41(1):23-30. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2006. PMID: 16619846 Review. Japanese.
Cited by
-
Regulated proteolytic processing of Reelin through interplay of tissue plasminogen activator (tPA), ADAMTS-4, ADAMTS-5, and their modulators.PLoS One. 2012;7(10):e47793. doi: 10.1371/journal.pone.0047793. Epub 2012 Oct 17. PLoS One. 2012. PMID: 23082219 Free PMC article.
-
Regulation of seizure spreading by neuroserpin and tissue-type plasminogen activator is plasminogen-independent.J Clin Invest. 2002 Jun;109(12):1571-8. doi: 10.1172/JCI14308. J Clin Invest. 2002. PMID: 12070304 Free PMC article.
-
Modulations of the neuronal trafficking of tissue-type plasminogen activator (tPA) influences glutamate release.Cell Death Dis. 2023 Jan 18;14(1):34. doi: 10.1038/s41419-022-05543-9. Cell Death Dis. 2023. PMID: 36650132 Free PMC article.
-
PKCδ-positive GABAergic neurons in the central amygdala exhibit tissue-type plasminogen activator: role in the control of anxiety.Mol Psychiatry. 2022 Apr;27(4):2197-2205. doi: 10.1038/s41380-022-01455-4. Epub 2022 Feb 10. Mol Psychiatry. 2022. PMID: 35145231
-
Passenger mutations and aberrant gene expression in congenic tissue plasminogen activator-deficient mouse strains.J Thromb Haemost. 2016 Aug;14(8):1618-28. doi: 10.1111/jth.13338. Epub 2016 Jun 20. J Thromb Haemost. 2016. PMID: 27079292 Free PMC article.
References
-
- Baranes D, Lederfein D, Huang YY, Chen M, Bailey CH, Kandel ER. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron. 1998;21:813–825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
