Selective blockade of mGlu5 metabotropic glutamate receptors is protective against methamphetamine neurotoxicity
- PMID: 11896153
- PMCID: PMC6758277
- DOI: 10.1523/JNEUROSCI.22-06-02135.2002
Selective blockade of mGlu5 metabotropic glutamate receptors is protective against methamphetamine neurotoxicity
Abstract
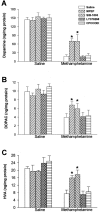
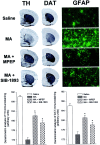
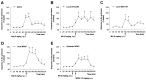
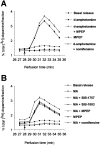
Methamphetamine (MA), a widely used drug of abuse, produces oxidative damage of nigrostriatal dopaminergic terminals. We examined the effect of subtype-selective ligands of metabotropic glutamate (mGlu) receptors on MA neurotoxicity in mice. MA (5 mg/kg, i.p.; injected three times, every 2 hr) induced, 5 d later, a substantial degeneration of striatal dopaminergic terminals associated with reactive gliosis. MA toxicity was primarily attenuated by the coinjection of the noncompetitive mGlu5 receptor antagonists 2-methyl-6-(phenylethynyl)pyridine and (E)-2-methyl-6-styrylpyridine both at 10 mg/kg, i.p.). In contrast, the mGlu1 receptor antagonist 7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester (10 mg/kg, i.p.), and the mGlu2/3 receptor agonist (-)-2-oxa-4-aminocyclo[3.1.0]hexane-4,6-dicarboxylic acid (1 mg/kg, i.p.), failed to affect MA toxicity. mGlu5 receptor antagonists reduced the production of reactive oxygen species but did not reduce the acute stimulation of dopamine release induced by MA both in striatal synaptosomes and in the striatum of freely moving mice. We conclude that endogenous activation of mGlu5 receptors enables the development of MA neurotoxicity and that mGlu5 receptor antagonists are neuroprotective without interfering with the primary mechanism of action of MA.
Figures

Similar articles
-
Alpha-1B adrenergic receptor knockout mice are protected against methamphetamine toxicity.J Neurochem. 2003 Jul;86(2):413-21. doi: 10.1046/j.1471-4159.2003.01867.x. J Neurochem. 2003. PMID: 12871582
-
Endogenous activation of mGlu5 metabotropic glutamate receptors contributes to the development of nigro-striatal damage induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice.J Neurosci. 2004 Jan 28;24(4):828-35. doi: 10.1523/JNEUROSCI.3831-03.2004. J Neurosci. 2004. PMID: 14749427 Free PMC article.
-
Neurokinin-1 (NK-1) receptor antagonists abrogate methamphetamine-induced striatal dopaminergic neurotoxicity in the murine brain.J Neurochem. 2002 Nov;83(3):613-22. doi: 10.1046/j.1471-4159.2002.01155.x. J Neurochem. 2002. PMID: 12390523
-
The role of striatal metabotropic glutamate receptors in degeneration of dopamine neurons: review article.Amino Acids. 2002;23(1-3):199-205. doi: 10.1007/s00726-001-0129-z. Amino Acids. 2002. PMID: 12373538 Review.
-
Substance P and cholecystokinin regulate neurochemical responses to cocaine and methamphetamine in the striatum.Life Sci. 2003 Jun 27;73(6):727-39. doi: 10.1016/s0024-3205(03)00393-x. Life Sci. 2003. PMID: 12801594 Review.
Cited by
-
The effects of locus coeruleus and norepinephrine in methamphetamine toxicity.Curr Neuropharmacol. 2013 Jan;11(1):80-94. doi: 10.2174/157015913804999522. Curr Neuropharmacol. 2013. PMID: 23814540 Free PMC article.
-
Serial exposure to ethanol drinking and methamphetamine enhances glutamate excitotoxicity.J Neurochem. 2019 Dec;151(6):749-763. doi: 10.1111/jnc.14861. Epub 2019 Oct 15. J Neurochem. 2019. PMID: 31478210 Free PMC article.
-
Molecular bases of methamphetamine-induced neurodegeneration.Int Rev Neurobiol. 2009;88:101-19. doi: 10.1016/S0074-7742(09)88005-7. Int Rev Neurobiol. 2009. PMID: 19897076 Free PMC article. Review.
-
Mechanisms of methamphetamine-induced dopaminergic neurotoxicity.AAPS J. 2006;8(2):E413-8. doi: 10.1007/BF02854914. AAPS J. 2006. PMID: 16808044 Free PMC article. Review.
-
Fine structure and biochemical mechanisms underlying nigrostriatal inclusions and cell death after proteasome inhibition.J Neurosci. 2003 Oct 1;23(26):8955-66. doi: 10.1523/JNEUROSCI.23-26-08955.2003. J Neurosci. 2003. PMID: 14523098 Free PMC article.
References
-
- Abekawa T, Ohmori T, Koyama T. Effects of repeated administration of a high dose of methamphetamine on dopamine and glutamate release in rat striatum and nucleus accumbens. Brain Res. 1994;643:276–281. - PubMed
-
- Alagarsamy S, Marino MJ, Rouse ST, Gereau IVRW, Heinemann SF, Conn PJ. Activation of NMDA receptors reverses desensitization of mGluR5 in native and recombinant systems. Nat Neurosci. 1999;2:234–240. - PubMed
-
- Albers DS, Sonsalla PK. Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: pharmacological profile of protective and nonprotective agents. J Pharmacol Exp Ther. 1995;275:1104–1114. - PubMed
-
- Ali SF, Newport GD, Holson RR, Slikker W, Jr, Bowyer JF. Low environmental temperature or pharmacological agents that produce hyperthermia decrease methamphetamine neurotoxicity in mice. Brain Res. 1994;658:33–38. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
