Expression of a variant form of the glutamate transporter GLT1 in neuronal cultures and in neurons and astrocytes in the rat brain
- PMID: 11896154
- PMCID: PMC2849837
- DOI: 10.1523/JNEUROSCI.22-06-02142.2002
Expression of a variant form of the glutamate transporter GLT1 in neuronal cultures and in neurons and astrocytes in the rat brain
Abstract
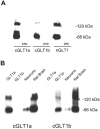
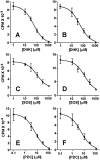
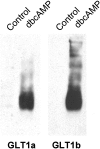
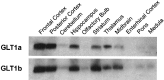
To identify glutamate transporters expressed in forebrain neurons, we prepared a cDNA library from rat forebrain neuronal cultures, previously shown to transport glutamate with high affinity and capacity. Using this library, we cloned two forms, varying in the C terminus, of the glutamate transporter GLT1. This transporter was previously found to be localized exclusively in astrocytes in the normal mature brain. Specific antibodies against the C-terminal peptides were used to show that forebrain neurons in culture express both GLT1a and GLT1b proteins. The pharmacological properties of glutamate transport mediated by GLT1a and GLT1b expressed in COS-7 cells and in neuronal cultures were indistinguishable. Both GLT1a and GLT1b were upregulated in astrocyte cultures by exposure to dibutyryl cAMP. We next investigated the expression of GLT1b in vivo. Northern blot analysis of forebrain RNA revealed two transcripts of approximately 3 and 11 kb that became more plentiful with developmental age. Immunoblot analysis showed high levels of expression in the cortex, hippocampus, striatum, thalamus, and midbrain. Pre-embedding electron microscopic immunocytochemistry with silver-enhanced immunogold detection was used to localize GLT1b in vivo. In the rat somatosensory cortex, GLT1b was clearly expressed in neurons in presynaptic terminals and dendritic shafts, as well as in astrocytes. The presence of GLT1b in neurons may offer a partial explanation for the observed uptake of glutamate by presynaptic terminals, for the preservation of input specificity at excitatory synapses, and may play a role in the pathophysiology of excitotoxicity.
Figures




References
-
- Asztely F, Erdemli G, Kullmann DM. Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake. Neuron. 1997;18:281–293. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
- NS 41883/NS/NINDS NIH HHS/United States
- R01 NS041091/NS/NINDS NIH HHS/United States
- P30 EY013079/EY/NEI NIH HHS/United States
- P30 HD018655/HD/NICHD NIH HHS/United States
- R01 EY013145/EY/NEI NIH HHS/United States
- R01 NS040753/NS/NINDS NIH HHS/United States
- NS 40753/NS/NINDS NIH HHS/United States
- NS41091/NS/NINDS NIH HHS/United States
- EY13145/EY/NEI NIH HHS/United States
- HD18655/HD/NICHD NIH HHS/United States
- EY13079/EY/NEI NIH HHS/United States
- R25 NS080686/NS/NINDS NIH HHS/United States
- R01 NS041883/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
