The mitochondrial toxin 3-nitropropionic acid induces striatal neurodegeneration via a c-Jun N-terminal kinase/c-Jun module
- PMID: 11896157
- PMCID: PMC6758250
- DOI: 10.1523/JNEUROSCI.22-06-02174.2002
The mitochondrial toxin 3-nitropropionic acid induces striatal neurodegeneration via a c-Jun N-terminal kinase/c-Jun module
Abstract
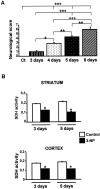
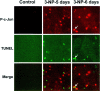
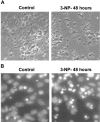
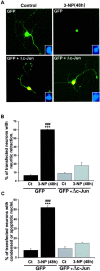
Impairments in mitochondrial energy metabolism are thought to be involved in most neurodegenerative diseases, including Huntington's disease (HD). Chronic administration of 3-nitropropionic acid (3-NP), a suicide inhibitor of succinate dehydrogenase, causes prolonged energy impairments and replicates most of the pathophysiological features of HD, including preferential striatal degeneration. In this study, we analyzed one of the mechanisms that could account for this selective 3-NP-induced striatal degeneration. In chronically 3-NP-infused rats, the time course of motor behavioral impairments and histological abnormalities was determined. Progressive alterations of motor performance occurred after 3 d. By histological analysis and terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end-labeling staining, we found a selective neurodegenerescence in the striatum, occurring first in its dorsolateral (DL) part. Activation of c-Jun N-terminal kinase (JNK) was analyzed from brain sections of these rats, using immunocytochemical detection of its phosphorylated form. Activation of JNK occurred progressively and selectively in the DL of the striatum and was followed by c-Jun activation and expression in the same striatal region. To elucidate the role of the JNK/c-Jun module in 3-NP-induced striatal degeneration, we then used primary striatal neurons in culture, in which we replicated neuronal death by application of 3-NP. We found strong nuclear translocation of activated JNK that was rapidly followed by phosphorylation of the transcription factor c-Jun. Overexpression of a dominant negative version of c-Jun, lacking its transactivation domain and phosphorylation sites for activated JNK, completely abolished 3-NP-induced striatal neurodegeneration. We thus conclude that a genetic program controlled by the JNK/c-Jun module is an important molecular event in 3-NP-induced striatal degeneration.
Figures





References
-
- Angel P, Hattori K, Smeal T, Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988;55:875–885. - PubMed
-
- Beal MF. Mitochondria, free radicals, and neurodegeneration. Curr Opin Neurobiol. 1996;6:661–666. - PubMed
-
- Beal MF. Energetics in the pathogenesis of neurodegenerative diseases. Trends Neurosci. 2000;23:298–304. - PubMed
-
- Behrens A, Sibilia M, Wagner EF. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat Genet. 1999;21:326–329. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
