Versican is upregulated in CNS injury and is a product of oligodendrocyte lineage cells
- PMID: 11896162
- PMCID: PMC6758262
- DOI: 10.1523/JNEUROSCI.22-06-02225.2002
Versican is upregulated in CNS injury and is a product of oligodendrocyte lineage cells
Abstract
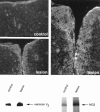
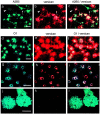
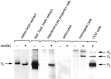
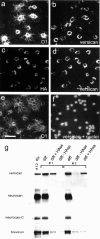
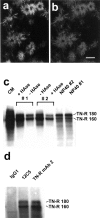
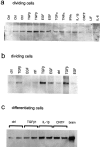
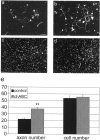
Chondroitin sulfate proteoglycan (CS-PG) expression is increased in response to CNS injury and limits the capacity for axonal regeneration. Previously we have shown that neurocan is one of the CS-PGs that is upregulated (Asher et al., 2000). Here we show that another member of the aggrecan family, versican, is also upregulated in response to CNS injury. Labeling of frozen sections 7 d after a unilateral knife lesion to the cerebral cortex revealed a clear increase in versican immunoreactivity around the lesion. Western blot analysis of extracts prepared from injured and uninjured tissue also revealed considerably more versican in the injured tissue extract. In vitro studies revealed versican to be a product of oligodendrocyte lineage cells (OLCs). Labeling was seen between the late A2B5-positive stage and the O1-positive pre-oligodendrocyte stage. Neither immature, bipolar A2B5-positive cells, nor differentiated, myelin-forming oligodendrocytes were labeled. The amount of versican in conditioned medium increased as these cells differentiated. Versican and tenascin-R colocalized in OLCs, and coimmunoprecipitation indicated that the two exist as a complex in oligodendrocyte-conditioned medium. Treatment of pre-oligodendrocytes with hyaluronidase led to the release of versican, indicating that its retention at the cell surface is dependent on hyaluronate (HA). In rat brain, approximately half of the versican is bound to hyaluronate. We also provide evidence of a role for CS-PGs in the axon growth-inhibitory properties of oligodendrocytes. Because large numbers of OLCs are recruited to CNS lesions, these results suggest that OLC-derived versican contributes to the inhospitable environment of the injured CNS.
Figures

References
-
- Asher R, Perides G, Vanderhaeghen J-J, Bignami A. Extracellular matrix of central nervous system white matter: demonstration of a hyaluronate-protein complex. J Neurosci Res. 1991;28:410–421. - PubMed
-
- Asher RA, Scheibe RJ, Keiser HD, Bignami A. On the existence of a cartilage-like proteoglycan and link proteins in the central nervous system. Glia. 1995;13:294–308. - PubMed
-
- Asher RA, Morgenstern DA, Adcock KH, Rogers JH, Fawcett JW. Versican is up-regulated in CNS injury and is a product of O-2A lineage cells. Soc Neurosci Abstr. 1999;25:750.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
