Lack of the cell-cycle inhibitor p27Kip1 results in selective increase of transit-amplifying cells for adult neurogenesis
- PMID: 11896165
- PMCID: PMC6758265
- DOI: 10.1523/JNEUROSCI.22-06-02255.2002
Lack of the cell-cycle inhibitor p27Kip1 results in selective increase of transit-amplifying cells for adult neurogenesis
Abstract
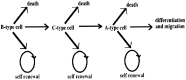
The subventricular zone (SVZ) is the largest germinal layer in the adult mammalian brain and comprises stem cells, transit-amplifying progenitors, and committed neuroblasts. Although the SVZ contains the highest concentration of dividing cells in the adult brain, the intracellular mechanisms controlling their proliferation have not been elucidated. We show here that loss of the cyclin-dependent kinase inhibitor p27Kip1 has very specific effects on a population of CNS progenitors responsible for adult neurogenesis. Using bromodeoxyuridine and [(3)H]thymidine incorporation to label cells in S phase and cell-specific markers and electron microscopy to identify distinct cell types, we compared the SVZ structure and proliferation characteristics of wild-type and p27Kip1-null mice. Loss of p27Kip1 had no effect on the number of stem cells but selectively increased the number of the transit-amplifying progenitors concomitantly with a reduction in the number of neuroblasts. We conclude that cell-cycle regulation of SVZ adult progenitors is remarkably cell-type specific, with p27Kip1 being a key regulator of the cell division of the transit-amplifying progenitors.
Figures

Similar articles
-
Increased neurogenesis in adult mCD24-deficient mice.J Neurosci. 2002 May 1;22(9):3594-607. doi: 10.1523/JNEUROSCI.22-09-03594.2002. J Neurosci. 2002. PMID: 11978835 Free PMC article.
-
Loss of p27Kip1 function results in increased proliferative capacity of oligodendrocyte progenitors but unaltered timing of differentiation.Development. 1999 Sep;126(18):4027-37. doi: 10.1242/dev.126.18.4027. Development. 1999. PMID: 10457012
-
Dopamine stimulation of postnatal murine subventricular zone neurogenesis via the D3 receptor.J Neurochem. 2010 Aug;114(3):750-60. doi: 10.1111/j.1471-4159.2010.06799.x. Epub 2010 May 6. J Neurochem. 2010. PMID: 20477937 Free PMC article.
-
Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain.Neuron. 2011 May 26;70(4):674-86. doi: 10.1016/j.neuron.2011.05.004. Neuron. 2011. PMID: 21609824 Free PMC article. Review.
-
Transit Amplifying Progenitors in the Cerebellum: Similarities to and Differences from Transit Amplifying Cells in Other Brain Regions and between Species.Cells. 2022 Feb 18;11(4):726. doi: 10.3390/cells11040726. Cells. 2022. PMID: 35203375 Free PMC article. Review.
Cited by
-
p27(KIP1) regulates neurogenesis in the rostral migratory stream and olfactory bulb of the postnatal mouse.J Neurosci. 2009 Mar 4;29(9):2902-14. doi: 10.1523/JNEUROSCI.4051-08.2009. J Neurosci. 2009. PMID: 19261886 Free PMC article.
-
CHD7 maintains neural stem cell quiescence and prevents premature stem cell depletion in the adult hippocampus.Stem Cells. 2015 Jan;33(1):196-210. doi: 10.1002/stem.1822. Stem Cells. 2015. PMID: 25183173 Free PMC article.
-
Intermediate progenitors are increased by lengthening of the cell cycle through calcium signaling and p53 expression in human neural progenitors.Mol Biol Cell. 2012 Apr;23(7):1167-80. doi: 10.1091/mbc.E11-06-0524. Epub 2012 Feb 9. Mol Biol Cell. 2012. PMID: 22323293 Free PMC article.
-
Patterns of p57Kip2 expression in embryonic rat brain suggest roles in progenitor cell cycle exit and neuronal differentiation.Dev Neurobiol. 2009 Jan;69(1):1-21. doi: 10.1002/dneu.20680. Dev Neurobiol. 2009. PMID: 18814313 Free PMC article.
-
A symphony of signals conducts early and late stages of adult neurogenesis.Neuropharmacology. 2010 May;58(6):865-76. doi: 10.1016/j.neuropharm.2010.01.010. Epub 2010 Jan 25. Neuropharmacology. 2010. PMID: 20097213 Free PMC article. Review.
References
-
- Alvarez-Buylla A, Vicario DS. Simple microcomputer system for mapping tissue sections with the light microscope. J Neurosci Methods. 1988;25:165–173. - PubMed
-
- Biebl M, Cooper CM, Winkler J, Kuhn HG. Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci Lett. 2000;291:17–20. - PubMed
-
- Casaccia-Bonnefil P, Hardy RJ, Teng KK, Levine JM, Koff A, Chao MV. Loss of p27Kip1 function results in increased proliferative capacity of oligodendrocyte progenitors but unaltered timing of differentiation. Development. 1999;126:4027–4037. - PubMed
-
- Caviness VS, Jr, Takahashi T, Nowakowki RS. The G1 restriction point as critical regulator of cortical neuronogenesis. Neurochem Res. 1999;24:497–506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
