Serotonin release evoked by tail nerve stimulation in the CNS of aplysia: characterization and relationship to heterosynaptic plasticity
- PMID: 11896169
- PMCID: PMC6758260
- DOI: 10.1523/JNEUROSCI.22-06-02299.2002
Serotonin release evoked by tail nerve stimulation in the CNS of aplysia: characterization and relationship to heterosynaptic plasticity
Abstract
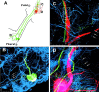
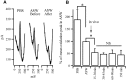
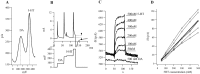
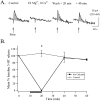
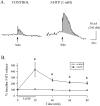
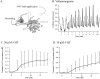
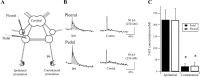
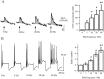
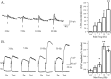
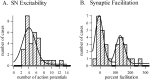
Considerable experimental evidence suggests that serotonin (5-HT) at sensory neuron-->motor neuron (SN-->MN) synapses, as well as other neuronal sites, contributes importantly to simple forms of learning such as sensitization and classical conditioning in Aplysia. However, the actual release of 5-HT in the CNS induced by sensitizing stimuli such as tail shock has not been directly demonstrated. In this study, we addressed this question by (1) immunohistochemically labeling central 5-HT processes and (2) directly measuring with chronoamperometry the release of 5-HT induced by pedal tail nerve (P9) shock onto tail SNs in the pleural ganglion and their synapses onto tail MNs in the pedal ganglion. We found that numerous 5-HT-immunoreactive fibers surround both the SN cell bodies in the pleural ganglion and SN axons in the pedal ganglion. Chronoamperometric detection of 5-HT performed with carbon fiber electrodes implanted in the vicinity of tail SN somata and synapses revealed an electrochemical 5-HT signal lasting approximately 40 sec after a brief shock of P9. 5-HT release was restricted to discrete subregions (modulatory fields) of the CNS, including the vicinity of tail SN soma and synapses ipsilateral to the stimulation. Increasing P9 shock frequency augmented the amplitude of the 5-HT signal and, in parallel, increased SN excitability and SN synaptic transmission onto tail MNs. However, the relationship between the amount of 5-HT release and the two forms of SN plasticity was not uniform: SN excitability increased in a graded manner with increased 5-HT release, whereas synaptic facilitation exhibited a highly nonlinear relationship. The development of chronoamperometric techniques in Aplysia now paves the way for a more complete understanding of the contribution of the serotonergic modulatory pathway to memory processing in this system.
Figures


Similar articles
-
Regulation of behavioral and synaptic plasticity by serotonin release within local modulatory fields in the CNS of Aplysia.J Neurosci. 2006 Dec 6;26(49):12682-93. doi: 10.1523/JNEUROSCI.3309-06.2006. J Neurosci. 2006. PMID: 17151271 Free PMC article.
-
Serotonin acts in the synaptic region of sensory neurons in Aplysia to enhance transmitter release.Neurosci Lett. 1989 Sep 25;104(1-2):235-40. doi: 10.1016/0304-3940(89)90360-1. Neurosci Lett. 1989. PMID: 2573016
-
Evolution of learning in three aplysiid species: differences in heterosynaptic plasticity contrast with conservation in serotonergic pathways.J Physiol. 2003 Jul 1;550(Pt 1):241-53. doi: 10.1113/jphysiol.2003.038356. Epub 2003 May 9. J Physiol. 2003. PMID: 12740422 Free PMC article.
-
Postsynaptic regulation of the development and long-term plasticity of Aplysia sensorimotor synapses in cell culture.J Neurobiol. 1994 Jun;25(6):666-93. doi: 10.1002/neu.480250608. J Neurobiol. 1994. PMID: 8071666 Review.
-
Multiple serotonergic mechanisms contributing to sensitization in aplysia: evidence of diverse serotonin receptor subtypes.Learn Mem. 2003 Sep-Oct;10(5):373-86. doi: 10.1101/lm.66103. Learn Mem. 2003. PMID: 14557610 Free PMC article. Review.
Cited by
-
Role of protein kinase C in the induction and maintenance of serotonin-dependent enhancement of the glutamate response in isolated siphon motor neurons of Aplysia californica.J Neurosci. 2009 Apr 22;29(16):5100-7. doi: 10.1523/JNEUROSCI.4149-08.2009. J Neurosci. 2009. PMID: 19386905 Free PMC article.
-
Inhibition of calcineurin facilitates the induction of memory for sensitization in Aplysia: requirement of mitogen-activated protein kinase.Proc Natl Acad Sci U S A. 2003 Apr 15;100(8):4861-6. doi: 10.1073/pnas.0830994100. Epub 2003 Apr 2. Proc Natl Acad Sci U S A. 2003. PMID: 12672952 Free PMC article.
-
Latent memory for sensitization in Aplysia.Learn Mem. 2006 Mar-Apr;13(2):224-9. doi: 10.1101/lm.111506. Learn Mem. 2006. PMID: 16585798 Free PMC article.
-
Quantitation of contacts among sensory, motor, and serotonergic neurons in the pedal ganglion of aplysia.Learn Mem. 2003 Sep-Oct;10(5):387-93. doi: 10.1101/lm.63903. Learn Mem. 2003. PMID: 14557611 Free PMC article.
-
Differential role of inhibition in habituation of two independent afferent pathways to a common motor output.Learn Mem. 2005 Jan-Feb;12(1):52-60. doi: 10.1101/lm.83405. Epub 2005 Jan 12. Learn Mem. 2005. PMID: 15647595 Free PMC article.
References
-
- Abrams TW, Castellucci VF, Camardo JS, Kandel ER, Lloyd PE. Two endogenous neuropeptides modulate the gill and siphon withdrawal reflex in Aplysia by presynaptic facilitation involving cAMP-dependent closure of a serotonin-sensitive potassium channel. Proc Natl Acad Sci USA. 1984;81:7956–7960. - PMC - PubMed
-
- Alberini C, Ghirardi M, Metz R, Kandel E. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell. 1994;76:1099–1114. - PubMed
-
- Barbas D, Zappulla J, Angers S, Bouvier M, Castellucci V, DesGroseillers L. Functional characterization of a novel serotonin receptor (5-HTap2) expressed in the CNS of Aplysia californica. J Neurochem. 2002;80:335–345. - PubMed
-
- Brabham DG, Fernandez RI, Levenson J, Eichberg J, Byrne JH, Eskin A. Diurnal regulation of serotonin synthesis and expression of sensitization in Aplysia. Soc Neurosci Abstr. 2000;26:1525.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
