Episodic bursting activity and response to excitatory amino acids in acutely dissociated gonadotropin-releasing hormone neurons genetically targeted with green fluorescent protein
- PMID: 11896170
- PMCID: PMC6758266
- DOI: 10.1523/JNEUROSCI.22-06-02313.2002
Episodic bursting activity and response to excitatory amino acids in acutely dissociated gonadotropin-releasing hormone neurons genetically targeted with green fluorescent protein
Abstract
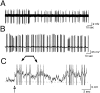
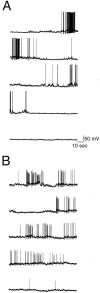
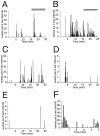
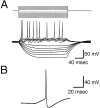
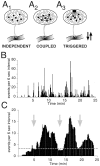
The gonadotropin-releasing hormone (GnRH) system, considered to be the final common pathway for the control of reproduction, has been difficult to study because of a lack of distinguishing characteristics and the scattered distribution of neurons. The development of a transgenic mouse in which the GnRH promoter drives expression of enhanced green fluorescent protein (EGFP) has provided the opportunity to perform electrophysiological studies of GnRH neurons. In this study, neurons were dissociated from brain slices prepared from prepubertal female GnRH-EGFP mice. Both current- and voltage-clamp recordings were obtained from acutely dissociated GnRH neurons identified on the basis of EGFP expression. Most isolated GnRH-EGFP neurons fired spontaneous action potentials (recorded in cell-attached or whole-cell mode) that typically consisted of brief bursts (2-20 Hz) separated by 1-10 sec. At more negative resting potentials, GnRH-EGFP neurons exhibited oscillations in membrane potential, which could lead to bursting episodes lasting from seconds to minutes. These bursting episodes were often separated by minutes of inactivity. Rapid application of glutamate or NMDA increased firing activity in all neurons and usually generated small inward currents (<15 pA), although larger currents were evoked in the remaining neurons. Both AMPA and NMDA receptors mediated the glutamate-evoked inward currents. These results suggest that isolated GnRH-EGFP neurons from juvenile mice can generate episodes of repetitive burst discharges that may underlie the pulsatile secretion of GnRH, and glutamatergic inputs may contribute to the activation of endogenous bursts.
Figures



Comment in
-
GnRH neurons and episodic bursting activity.Trends Endocrinol Metab. 2002 Dec;13(10):409-10. doi: 10.1016/s1043-2760(02)00698-7. Trends Endocrinol Metab. 2002. PMID: 12431832 No abstract available.
Similar articles
-
Spike-dependent depolarizing afterpotentials contribute to endogenous bursting in gonadotropin releasing hormone neurons.Neuroscience. 2005;134(1):295-300. doi: 10.1016/j.neuroscience.2005.03.047. Neuroscience. 2005. PMID: 15961246
-
Whole-cell recordings from preoptic/hypothalamic slices reveal burst firing in gonadotropin-releasing hormone neurons identified with green fluorescent protein in transgenic mice.Endocrinology. 2000 Oct;141(10):3731-6. doi: 10.1210/endo.141.10.7690. Endocrinology. 2000. PMID: 11014229
-
Frequency-dependent recruitment of fast amino acid and slow neuropeptide neurotransmitter release controls gonadotropin-releasing hormone neuron excitability.J Neurosci. 2011 Feb 16;31(7):2421-30. doi: 10.1523/JNEUROSCI.5759-10.2011. J Neurosci. 2011. PMID: 21325509 Free PMC article.
-
Understanding calcium homeostasis in postnatal gonadotropin-releasing hormone neurons using cell-specific Pericam transgenics.Cell Calcium. 2012 Mar-Apr;51(3-4):267-76. doi: 10.1016/j.ceca.2011.11.005. Epub 2011 Dec 15. Cell Calcium. 2012. PMID: 22177387 Review.
-
17β-oestradiol regulation of gonadotrophin-releasing hormone neuronal excitability.J Neuroendocrinol. 2012 Jan;24(1):122-30. doi: 10.1111/j.1365-2826.2011.02160.x. J Neuroendocrinol. 2012. PMID: 21592235 Free PMC article. Review.
Cited by
-
Ion channels and information processing in GnRH neuron dendrites.Channels (Austin). 2013 May-Jun;7(3):135-45. doi: 10.4161/chan.24228. Epub 2013 Mar 21. Channels (Austin). 2013. PMID: 23519241 Free PMC article. Review.
-
The SK channel blocker apamin inhibits slow afterhyperpolarization currents in rat gonadotropin-releasing hormone neurones.J Physiol. 2006 Jul 15;574(Pt 2):431-42. doi: 10.1113/jphysiol.2006.110155. Epub 2006 Apr 20. J Physiol. 2006. PMID: 16627563 Free PMC article.
-
Modulation of Gonadotropin-Releasing Hormone Neuron Activity and Secretion in Mice by Non-peptide Neurotransmitters, Gasotransmitters, and Gliotransmitters.Front Endocrinol (Lausanne). 2019 May 22;10:329. doi: 10.3389/fendo.2019.00329. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31178828 Free PMC article. Review.
-
Dual-phenotype GABA/glutamate neurons in adult preoptic area: sexual dimorphism and function.J Neurosci. 2004 Sep 15;24(37):8097-105. doi: 10.1523/JNEUROSCI.2267-04.2004. J Neurosci. 2004. PMID: 15371511 Free PMC article.
-
Age affects spontaneous activity and depolarizing afterpotentials in isolated gonadotropin-releasing hormone neurons.Endocrinology. 2008 Oct;149(10):4938-47. doi: 10.1210/en.2008-0308. Epub 2008 Jun 26. Endocrinology. 2008. PMID: 18583421 Free PMC article.
References
-
- Abud R, Smith MS. Do GnRH neurons express the gene for the NMDA receptor? Brain Res. 1995;690:117–120. - PubMed
-
- Andrew RD, Dudek FE. Burst discharge in mammalian neuroendocrine cells involves an intrinsic regenerative mechanism. Science. 1983;221:1050–1052. - PubMed
-
- Andrew RD, Dudek FE. Analysis of intracellularly recorded phasic bursting by mammalian neuroendocrine cells. J Neurophysiol. 1984;51:552–566. - PubMed
-
- Andrews WW, Ojeda SR. The control of LH release in prepubertal female rats: indirect evidence for an enhanced ability of the hypothalamus to release LHRH as the pituitary responsiveness to LHRH declines. J Endocrinol. 1978;78:281–282. - PubMed
-
- Andrews WW, Mizejewski GJ, Ojeda SR. Development of estradiol positive feedback on LH release in the female rat: a quantitative study. Endocrinology. 1981;109:1404–1413. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
