Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats
- PMID: 11896173
- PMCID: PMC6758267
- DOI: 10.1523/JNEUROSCI.22-06-02343.2002
Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats
Abstract
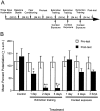
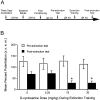
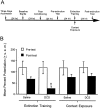
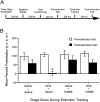
NMDA receptor antagonists block conditioned fear extinction when injected systemically and also when infused directly into the amygdala. Here we evaluate the ability of D-cycloserine (DCS), a partial agonist at the strychnine-insensitive glycine-recognition site on the NMDA receptor complex, to facilitate conditioned fear extinction after systemic administration or intra-amygdala infusions. Rats received 10 pairings of a 3.7 sec light and a 0.4 mA footshock (fear conditioning). Fear-potentiated startle (increased startle in the presence vs the absence of the light) was subsequently measured before and after 30, 60, or 90 presentations of the light without shock (extinction training). Thirty non-reinforced light presentations produced modest extinction, and 60 or 90 presentations produced nearly complete extinction (experiment 1). DCS injections (3.25, 15, or 30 mg/kg) before 30 non-reinforced light exposures dose-dependently enhanced extinction (experiment 2) but did not influence fear-potentiated startle in rats that did not receive extinction training (experiment 3). These effects were blocked by HA-966, an antagonist at the glycine-recognition site (experiment 4). Neither DCS nor HA-966 altered fear-potentiated startle when injected before testing (experiment 5). The effect of systemic administration was mimicked by intra-amygdala DCS (10 microg/side) infusions (experiment 6). These results indicate that treatments that promote NMDA receptor activity after either systemic or intra-amygdala administration promote the extinction of conditioned fear.
Figures
References
-
- Anthony EW, Nevins ME. Anxiolytic-like effects of N-methyl-d-aspartate-associated glycine receptor ligands in the rat potentiated startle test. Eur J Pharmacol. 1993;250:317–324. - PubMed
-
- Baker JD, Azorlosa JL. The NMDA antagonist MK-801 blocks the extinction of Pavlovian fear conditioning. Behav Neurosci. 1996;110:618–620. - PubMed
-
- Bouton ME, Bolles RC. Context, event-memories, and extinction. Lawrence Erlbaum Associates; Hillsdale, NJ: 1985.
-
- Bouton ME, Mineka S, Barlow DH. A modern learning theory perspective on the etiology of panic disorder. Psychol Rev. 2001;108:4–32. - PubMed
-
- Cassella JV, Harty PT, Davis M. Fear conditioning, pre-pulse inhibition, and drug modulation of a short latency startle response measure electromyographically from neck muscles in the rat. Physiol Behav. 1986;36:1187–1191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
