Autotransplantation for advanced lymphoma and Hodgkin's disease followed by post-transplant rituxan/GM-CSF or radiotherapy and consolidation chemotherapy
- PMID: 11896427
- PMCID: PMC7091694
- DOI: 10.1038/sj.bmt.1703363
Autotransplantation for advanced lymphoma and Hodgkin's disease followed by post-transplant rituxan/GM-CSF or radiotherapy and consolidation chemotherapy
Abstract
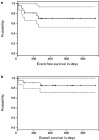
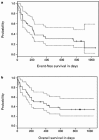
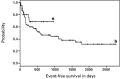
Disease relapse occurs in 50% or more of patients who are autografted for relapsed or refractory lymphoma (NHL) or Hodgkin's disease (HD). The administration of non-cross-resistant therapies during the post-transplant phase could possibly control residual disease and delay or prevent its progression. To test this approach, 55 patients with relapsed/refractory or high-risk NHL or relapsed/refractory HD were enrolled in the following protocol: stem cell mobilization: cyclophosphamide (4.5 g/m(2)) + etoposide (2.0 g/m(2)) followed by GM-CSF or G-CSF; high-dose therapy: gemcitabine (1.0 g/m(2)) on day -5, BCNU (300 mg/m(2)) + gemcitabine (1.0 g/m(2)) on day -2, melphalan (140 mg/m(2)) on day -1, blood stem cell infusion on day 0; post-transplant immunotherapy (B cell NHL): rituxan (375 mg/m(2)) weekly for 4 weeks + GM-CSF (250 microg thrice weekly) (weeks 4-8); post-transplant involved-field radiotherapy (HD): 30-40 Gy to pre-transplant areas of disease (weeks 4-8); post-transplant consolidation chemotherapy (all patients): dexamethasone (40 mg daily)/cyclophosphamide (300 mg/m(2)/day)/etoposide (30 mg/m(2)/day)/cisplatin (15 mg/m(2)/day) by continuous intravenous infusion for 4 days + gemcitabine (1.0 g/m(2), day 3) (months 3 + 9) alternating with dexamethasone/paclitaxel (135 mg/m(2))/cisplatin (75 mg/m(2)) (months 6 + 12). Of the 33 patients with B cell lymphoma, 14 had primary refractory disease (42%), 12 had relapsed disease (36%) and seven had high-risk disease in first CR (21%). For the entire group, the 2-year Kaplan-Meier event-free survival (EFS) and overall survival (OS) were 30% and 35%, respectively, while six of 33 patients (18%) died before day 100 from transplant-related complications. The rituxan/GM-CSF phase was well-tolerated by the 26 patients who were treated and led to radiographic responses in seven patients; an eighth patient with a blastic variant of mantle-cell lymphoma had clearance of marrow involvement after rituxan/GM-CSF. Of the 22 patients with relapsed/refractory HD (21 patients) or high-risk T cell lymphoblastic lymphoma (one patient), the 2-year Kaplan-Meier EFS and OS were 70% and 85%, respectively, while two of 22 patients (9%) died before day 100 from transplant-related complications. Eight patients received involved field radiation and seven had radiographic responses within the treatment fields. A total of 72 courses of post-transplant consolidation chemotherapy were administered to 26 of the 55 total patients. Transient grade 3-4 myelosuppression was common and one patient died from neutropenic sepsis, but no patients required an infusion of backup stem cells. After adjustment for known prognostic factors, the EFS for the cohort of HD patients was significantly better than the EFS for an historical cohort of HD patients autografted after BEAC (BCNU/etoposide/cytarabine/cyclophosphamide) without consolidation chemotherapy (P = 0.015). In conclusion, post-transplant consolidation therapy is feasible and well-tolerated for patients autografted for aggressive NHL and HD and may be associated with improved progression-free survival particularly for patients with HD.
Figures

Similar articles
-
Phase II study of immunomodulation with granulocyte-macrophage colony-stimulating factor, interleukin-2, and rituximab following autologous stem cell transplant in patients with relapsed or refractory lymphomas.Leuk Lymphoma. 2010 Jul;51(7):1241-50. doi: 10.3109/10428194.2010.486876. Leuk Lymphoma. 2010. PMID: 20496994 Clinical Trial.
-
High-dose therapy followed by autologous peripheral-blood stem-cell transplantation for patients with Hodgkin's disease and non-Hodgkin's lymphoma using unprimed and granulocyte colony-stimulating factor-mobilized peripheral-blood stem cells.J Clin Oncol. 1994 Oct;12(10):2176-86. doi: 10.1200/JCO.1994.12.10.2176. J Clin Oncol. 1994. PMID: 7523609 Clinical Trial.
-
Phase I/II study of high-dose cyclophosphamide, etoposide and cisplatin followed by autologous bone marrow or peripheral blood stem cell transplantation in patients with poor prognosis Hodgkin's disease or non-Hodgkin's lymphoma.Bone Marrow Transplant. 1993 Oct;12(4):337-45. Bone Marrow Transplant. 1993. PMID: 7506091 Clinical Trial.
-
Immunotherapy with rituximab following high-dose therapy and autologous stem-cell transplantation for mantle cell lymphoma.Semin Oncol. 2002 Feb;29(1 Suppl 2):56-69. Semin Oncol. 2002. PMID: 11842390 Review.
-
Treatment of relapsed aggressive lymphomas: regimens with and without high-dose therapy and stem cell rescue.Cancer Chemother Pharmacol. 2002 May;49 Suppl 1:S13-20. doi: 10.1007/s00280-002-0447-1. Epub 2002 Apr 12. Cancer Chemother Pharmacol. 2002. PMID: 12042984 Review.
Cited by
-
Clinical uses of GM-CSF, a critical appraisal and update.Biologics. 2008 Mar;2(1):13-27. doi: 10.2147/btt.s1355. Biologics. 2008. PMID: 19707424 Free PMC article.
-
Granisetron in the control of radiotherapy-induced nausea and vomiting: a comparison with other antiemetic therapies.Support Care Cancer. 2005 Sep;13(9):671-8. doi: 10.1007/s00520-004-0766-3. Epub 2005 Jul 26. Support Care Cancer. 2005. PMID: 16044252 Review.
-
Activation of peripheral-blood granulocytes is strongly correlated with patient outcome after immunotherapy with anti-GD2 monoclonal antibody and granulocyte-macrophage colony-stimulating factor.J Clin Oncol. 2012 Feb 1;30(4):426-32. doi: 10.1200/JCO.2011.37.6236. Epub 2011 Dec 27. J Clin Oncol. 2012. PMID: 22203761 Free PMC article. Clinical Trial.
-
The role of rituximab in autologous and allogeneic hematopoietic stem cell transplantation for non-Hodgkin's lymphoma.Curr Hematol Malig Rep. 2006 Dec;1(4):220-9. doi: 10.1007/s11899-006-0003-x. Curr Hematol Malig Rep. 2006. PMID: 20425317 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

