Nonviral gene transfer strategies for the vasculature
- PMID: 11896558
- PMCID: PMC4403639
- DOI: 10.1038/sj/mn/7800120
Nonviral gene transfer strategies for the vasculature
Abstract
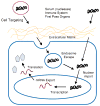
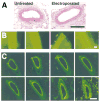
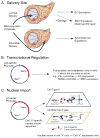
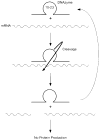
Major attention has been focused on the development of gene therapy approaches for the treatment of vascular diseases. In this review, we focus on an alternative use of gene therapy: the use of genetic means to study vascular cell biology and physiology. Both viral and nonviral gene transfer strategies have limitations, but because of the overwhelming inflammatory responses associated with the use of viral vectors, nonviral gene transfer methods are likely to be used more abundantly for future applications in the vasculature. Researchers have made great strides in the advancement of gene delivery to the vasculature in vivo. However, the efficiency of gene transfer seen with most nonviral approaches has been exceedingly low. We discuss how to circumvent and take advantage of a number of the barriers that limit efficient gene delivery to the vasculature to achieve high-level gene expression in appropriate cell types within the vessel wall. With such levels of expression, gene transfer offers the ability to alter pathways at the molecular level by genetically modulating the activity of a gene product, thus obviating the need to rely on pharmacological agents and their foreseen and unforeseen side effects. This genetic ability to alter distinct gene products within a signaling or biosynthetic pathway or to alter structural interactions within and between cells is extremely useful and technologically possible today. Hopefully, with the availability of these tools, new advances in cardiovascular physiology will emerge.
Figures


Similar articles
-
Nonviral vectors in the new millennium: delivery barriers in gene transfer.Hum Gene Ther. 2001 May 20;12(8):861-70. doi: 10.1089/104303401750195836. Hum Gene Ther. 2001. PMID: 11387052 Review.
-
Virally mediated gene transfer to the vasculature.Microcirculation. 2002 Jan;9(1):23-33. doi: 10.1038/sj.mn.7800119. Microcirculation. 2002. PMID: 11896557 Review.
-
In vivo electroporation: a powerful and convenient means of nonviral gene transfer to tissues of living animals (Review).Int J Mol Med. 1998 Jan;1(1):55-62. doi: 10.3892/ijmm.1.1.55. Int J Mol Med. 1998. PMID: 9852198 Review.
-
Gene therapy for hearing loss: Current status and future prospects of non-viral vector delivery systems.Hear Res. 2024 Nov;453:109130. doi: 10.1016/j.heares.2024.109130. Epub 2024 Oct 17. Hear Res. 2024. PMID: 39427589 Review.
-
Nonviral gene therapy.Curr Gene Ther. 2001 Jul;1(2):201-26. doi: 10.2174/1566523013348814. Curr Gene Ther. 2001. PMID: 12108955 Review.
Cited by
-
Nonviral gene transfer to skeletal, smooth, and cardiac muscle in living animals.Am J Physiol Cell Physiol. 2005 Aug;289(2):C233-45. doi: 10.1152/ajpcell.00613.2004. Am J Physiol Cell Physiol. 2005. PMID: 16002623 Free PMC article. Review.
-
Ultrasound‑targeted microbubbles combined with a peptide nucleic acid binding nuclear localization signal mediate transfection of exogenous genes by improving cytoplasmic and nuclear import.Mol Med Rep. 2017 Dec;16(6):8819-8825. doi: 10.3892/mmr.2017.7681. Epub 2017 Oct 2. Mol Med Rep. 2017. PMID: 28990051 Free PMC article.
-
Intracellular trafficking of nucleic acids.Expert Opin Drug Deliv. 2004 Nov;1(1):127-40. doi: 10.1517/17425247.1.1.127. Expert Opin Drug Deliv. 2004. PMID: 16296725 Free PMC article. Review.
-
Cell-specific targeting strategies for electroporation-mediated gene delivery in cells and animals.J Membr Biol. 2013 Oct;246(10):737-44. doi: 10.1007/s00232-013-9534-y. Epub 2013 Mar 24. J Membr Biol. 2013. PMID: 23525583 Free PMC article. Review.
-
Electroporation of the vasculature and the lung.DNA Cell Biol. 2003 Dec;22(12):797-806. doi: 10.1089/104454903322625000. DNA Cell Biol. 2003. PMID: 14683590 Free PMC article. Review.
References
-
- Anderson WF. Human gene therapy. Science. 1992;256:808–813. - PubMed
-
- Armeanu S, Pelisek J, Krausz E, Fuchs A, Groth D, Curth R, Keil O, Quilici J, Rolland PH, Reszka R, Nikol S. Optimization of nonviral gene transfer of vascular smooth muscle cells in vitro and in vivo. Mol Ther. 2000;1:366–375. - PubMed
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols Mol Biol. New York: John Wiley & Sons; 1994.
-
- Barron LG, Uyechi LS, Szoka FC., Jr Cationic lipids are essential for gene delivery mediated by intravenous administration of lipoplexes. Gene Ther. 1999;6:1179–1183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

