Deregulated expression of the human tumor marker CEA and CEA family member CEACAM6 disrupts tissue architecture and blocks colonocyte differentiation
- PMID: 11896570
- PMCID: PMC1550325
- DOI: 10.1038/sj.neo.7900201
Deregulated expression of the human tumor marker CEA and CEA family member CEACAM6 disrupts tissue architecture and blocks colonocyte differentiation
Abstract
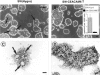
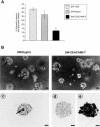
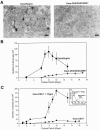
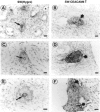
Human carcinoembryonic antigen (CEA) and the CEA family member CEACAM6 (formerly nonspecific cross-reacting antigen [NCA]) function in vitro, at least, as homotypic intercellular adhesion molecules and, in model systems, can block the terminal differentiation and anoikis of several different cell types. We have recently demonstrated that the increased cell surface levels of CEA and CEACAM6 in purified human colonocytes from freshly excised, well to poorly differentiated colon carcinomas are inversely correlated with the degree of cellular differentiation. Thus, deregulated expression of CEA/CEACAM6 could directly contribute to colon tumorigenesis by the inhibition of terminal differentiation and anoikis. Evidence against this view includes the common observation of increased CEA/CEACAM6 expression as normal colonocytes differentiate in their migration up colonic crypt walls. We report here the direct effects of deregulated overexpression of CEA/CEACAM6, at levels observed in colorectal carcinomas, on the differentiation of two human colonic cell lines, SW-1222 and Caco-2. Stable transfectants of both of these cell lines that constitutively express 10- to 30-fold higher cell surface levels of CEA/CEACAM6 than endogenous levels failed to polarize and differentiate into glandular structures in monolayer or 3D culture or to form colonic crypts in a tissue architecture assay in nude mice. In addition, these transfectants were found to exhibit increased tumorigenicity in nude mice. These results thus support the contention that deregulated overexpression of CEA and CEACAM6 could provide a tumorigenic contribution to colon carcinogenesis.
Figures



References
-
- Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. - PubMed
-
- Boucher D, Cournoyer D, Stanners CP, Fuks A. Studies on the control of gene expression of the carcinoembryonic antigen family in human tissue. Cancer Res. 1989;49:847–852. - PubMed
-
- Zimmer R, Thomas P. Mutations in the carcinoembryonic antigen gene in colorectal cancer patients: implications on liver metastasis. Cancer Res. 2001;61:2822–2826. - PubMed
-
- Chevinsky AH. CEA in tumors of other than colorectal origin. Semin Surg Oncol. 1991;7:162–166. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
