Identification and preliminary characterization of mouse Adam33
- PMID: 11897009
- PMCID: PMC88886
- DOI: 10.1186/1471-2156-3-2
Identification and preliminary characterization of mouse Adam33
Abstract
Background: The metalloprotease-disintegrin family, or ADAM, proteins, are implicated in cell-cell interactions, cell fusion, and cell signaling, and are widely distributed among metazoan phyla. Orthologous relationships have been defined for a few ADAM proteins including ADAM10 (Kuzbanian), and ADAM17 (TACE), but evolutionary relationships are not clear for the majority of family members. Human ADAM33 refers to a testis cDNA clone that does not contain a complete open reading frame, but portions of the predicted protein are similar to Xenopus laevis ADAM13.
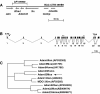
Results: In a 48 kb region of mouse DNA adjacent to the Attractin gene on mouse chromosome 2, we identified sequences very similar to human ADAM33. A full-length mouse cDNA was identified by a combination of gene prediction programs and RT-PCR, and the probable full-length human cDNA was identified by comparison to human genomic sequence in the homologous region on chromosome 20p13. Mouse ADAM33 is 44% identical to Xenopus laevis ADAM13, however a phylogenetic alignment and consideration of functional domains suggests that the two genes are not orthologous. Mouse Adam33 is widely expressed, most highly in the adult brain, heart, kidney, lung and testis.
Conclusions: While mouse ADAM33 is similar to Xenopus ADAM13 in sequence, further examination of its embryonic expression pattern, catalytic activity and protein interactions will be required to assess the functional relationship between these two proteins. Adam33 is expressed in the mouse adult brain and could play a role in complex processes that require cell-cell communication.
Figures



Similar articles
-
The role of ADAM33 in the pathogenesis of asthma.Springer Semin Immunopathol. 2004 Feb;25(3-4):361-75. doi: 10.1007/s00281-003-0153-z. Epub 2003 Nov 15. Springer Semin Immunopathol. 2004. PMID: 14999429 Review.
-
Identification and characterization of novel mouse and human ADAM33s with potential metalloprotease activity.Gene. 2002 Jan 9;282(1-2):227-36. doi: 10.1016/s0378-1119(01)00818-6. Gene. 2002. PMID: 11814695
-
PACSIN2 is a regulator of the metalloprotease/disintegrin ADAM13.Dev Biol. 2000 Nov 1;227(1):197-210. doi: 10.1006/dbio.2000.9871. Dev Biol. 2000. PMID: 11076687
-
Identification and characterization of ADAM32 with testis-predominant gene expression.Gene. 2003 Jan 30;304:151-62. doi: 10.1016/s0378-1119(02)01202-7. Gene. 2003. PMID: 12568724
-
Alpha-secretase activity of the disintegrin metalloprotease ADAM 10. Influences of domain structure.Ann N Y Acad Sci. 2000;920:215-22. doi: 10.1111/j.1749-6632.2000.tb06925.x. Ann N Y Acad Sci. 2000. PMID: 11193153 Review.
Cited by
-
Functions of 'A disintegrin and metalloproteases (ADAMs)' in the mammalian nervous system.Cell Mol Life Sci. 2019 Aug;76(16):3055-3081. doi: 10.1007/s00018-019-03173-7. Epub 2019 Jun 24. Cell Mol Life Sci. 2019. PMID: 31236626 Free PMC article. Review.
-
Increased expression of ADAM33 protein in asthmatic patients as compared to non-asthmatic controls.Indian J Med Res. 2013 Mar;137(3):507-14. Indian J Med Res. 2013. PMID: 23640557 Free PMC article.
-
Identification of novel polymorphisms in the Adam33 gene.J Hum Genet. 2003;48(5):278-281. doi: 10.1007/s10038-003-0019-1. Epub 2003 Apr 15. J Hum Genet. 2003. PMID: 12768445
-
ADAM 33 and its association with airway remodeling and hyperresponsiveness in asthma.Clin Rev Allergy Immunol. 2004 Aug;27(1):23-34. doi: 10.1385/CRIAI:27:1:023. Clin Rev Allergy Immunol. 2004. PMID: 15347848 Review.
-
The role of ADAM33 in the pathogenesis of asthma.Springer Semin Immunopathol. 2004 Feb;25(3-4):361-75. doi: 10.1007/s00281-003-0153-z. Epub 2003 Nov 15. Springer Semin Immunopathol. 2004. PMID: 14999429 Review.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

