Plant glutathione transferases
- PMID: 11897031
- PMCID: PMC139027
- DOI: 10.1186/gb-2002-3-3-reviews3004
Plant glutathione transferases
Abstract
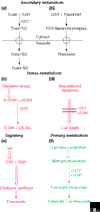
The soluble glutathione transferases (GSTs, EC 2.5.1.18) are encoded by a large and diverse gene family in plants, which can be divided on the basis of sequence identity into the phi, tau, theta, zeta and lambda classes. The theta and zeta GSTs have counterparts in animals but the other classes are plant-specific and form the focus of this article. The genome of Arabidopsis thaliana contains 48 GST genes, with the tau and phi classes being the most numerous. The GST proteins have evolved by gene duplication to perform a range of functional roles using the tripeptide glutathione (GSH) as a cosubstrate or coenzyme. GSTs are predominantly expressed in the cytosol, where their GSH-dependent catalytic functions include the conjugation and resulting detoxification of herbicides, the reduction of organic hydroperoxides formed during oxidative stress and the isomerization of maleylacetoacetate to fumarylacetoacetate, a key step in the catabolism of tyrosine. GSTs also have non-catalytic roles, binding flavonoid natural products in the cytosol prior to their deposition in the vacuole. Recent studies have also implicated GSTs as components of ultraviolet-inducible cell signaling pathways and as potential regulators of apoptosis. Although sequence diversification has produced GSTs with multiple functions, the structure of these proteins has been highly conserved. The GSTs thus represent an excellent example of how protein families can diversify to fulfill multiple functions while conserving form and structure.
Figures




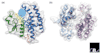
References
-
- Wilce MCJ, Parker MW. Structure and function of glutathione S-transferases. Biochim Biophys Acta. 1994;1205:1–18. A comprehensive review of mammalian GSTs. - PubMed
-
- Edwards R, Dixon DP. The role of glutathione transferases in herbicide metabolism. In: Cobb AH, Kirkwood RC, editor. In Herbicides and their mechanisms of action Edited by Cobb AH, Kirkwood RC Sheffield: Sheffield Academic Press, Sheffield: Sheffield Academic Press; 2000. pp. 33–71. A detailed review of the importance of glutathione conjugation in herbicide metabolism and selectivity in crops and weeds, including a description of the GSTs involved in catalyzing these reactions.
-
- Sheehan D, Meade G, Foley VM, Dowd CA. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J. 2001;360:1–16. One of the most recent reviews on GSTs, encompassing GSTs from insects, bacteria and plants. - PMC - PubMed
-
- Armstrong RN. Structure, catalytic mechanism, and evolution of the glutathione transferases. Chem Res Toxicol. 1997;10:2–18. A useful review of GSTs from an evolutionary perspective. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

