Development of a whole-cell assay for peptidoglycan biosynthesis inhibitors
- PMID: 11897573
- PMCID: PMC127110
- DOI: 10.1128/AAC.46.4.943-946.2002
Development of a whole-cell assay for peptidoglycan biosynthesis inhibitors
Abstract
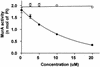
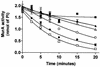
Osmotically stabilized Escherichia coli cells subjected to freezing and thawing were utilized as the source of enzymes for a peptidoglycan pathway assay that can be used to simultaneously test all targets of the committed steps of cell wall biosynthesis. The use of (14)C-labeled UDP-N-acetylglucosamine (UDP-GlcNAc) as a substrate allows the direct detection of cross-linked peptidoglycan formed. The assay was validated with known antibiotics. Fosfomycin was the strongest inhibitor of the pathway assay, with a 50% inhibitory concentration of 1 microM. Flavomycin, bacitracin, vancomycin, D-cycloserine, penicillin G, and ampicillin also inhibited formation of radiolabeled peptidoglycan by the E. coli cells. Screening of compounds identified two inhibitors of the pathway, Cpd1 and Cpd2. Subsequent tests with a biochemical assay utilizing purified enzyme implicated UDP-GlcNAc enolpyruvyl transferase (MurA) as the target of Cpd1. This compound inhibits the first enzyme of the pathway in a time-dependent manner. Moreover, enzyme inactivation is dependent on preincubation in the presence of UDP-GlcNAc, which forms a complex with MurA, exposing its active site. Cpd1 also displayed antimicrobial activity against a panel of microorganisms. The pathway assay used in conjunction with assays for individual enzymes provides an efficient means of detecting and characterizing novel antimicrobial agents.
Figures



References
-
- Amsterdan, D. 1966. Susceptibility testing of antimicrobials in liquid medium, p. 52-111. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. Williams & Wilkins, Baltimore, Md.
-
- Bernhardt, T. G., D. K. Struck, and R. Young. 2001. The lysis protein E of φX174 is a specific inhibitor of the MraY-catalyzed step in peptidoglycan synthesis. J. Biol. Chem. 276:6093-6097. - PubMed
-
- Bernhardt, T. G., I.-N. Wang, D. K. Struck, and R. Young. 2001. A protein antibiotic in the phage Qβ virion: diversity in lysis targets. Science 292:2326-2329. - PubMed
-
- Branstrom, A. A., S. Midha, C. B. Longley, K. Han, E. R. Baizman, and H. R. Axelrod. 2000. Assay for identification of inhibitors for bacterial MraY translocase and MurG transferase. Anal. Biochem. 280:315-319. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

