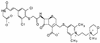In vitro and in vivo activities of a novel cephalosporin, BMS-247243, against methicillin-resistant and -susceptible staphylococci
- PMID: 11897577
- PMCID: PMC127082
- DOI: 10.1128/AAC.46.4.971-976.2002
In vitro and in vivo activities of a novel cephalosporin, BMS-247243, against methicillin-resistant and -susceptible staphylococci
Abstract
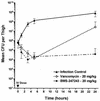
The recent emergence of methicillin-resistant Staphylococcus aureus (MRSA) with decreased susceptibility to vancomycin has intensified the search for alternative therapies for the treatment of infections caused by this organism. One approach has been to identify a beta-lactam with improved affinity for PBP 2a, the target enzyme responsible for methicillin resistance in staphylococci. BMS-247243 is such a candidate, with MICs that inhibit 90% of isolates tested (MIC(90)s) of 4, 2, and 8 microg/ml for methicillin-resistant strains of S. aureus, S. epidermidis, and S. haemolyticus, respectively, as determined on plates with Mueller-Hinton agar and 2% NaCl. The BMS-247243 MICs for MRSA were minimally affected by the susceptibility testing conditions (inoculum size, prolonged incubation, addition of salt to the test medium) or by staphylococcal beta-lactamases. BMS-247243 MIC(90)s for methicillin-susceptible staphylococcal species ranged from < or = 0.25 to 1 microg/ml. The BMS-247243 MIC(90) for beta-lactamase-producing S. aureus strains was fourfold higher than that for beta-lactamase-nonproducing strains. BMS-247243 is hydrolyzed by staphylococcal beta-lactamases at 4.5 to 26.2% of the rates measured for cephaloridine. The affinity of BMS-247243 for PBP 2a was >100-fold better than that of methicillin or cefotaxime. BMS-247243 is bactericidal for MRSA, killing the bacteria twice as fast as vancomycin. These in vitro activities of BMS-247243 correlated with its in vivo efficacy against infections in animals, including the neutropenic murine thigh and rabbit endocarditis models involving MRSA strains. In conclusion, BMS-247243 has in vitro and in vivo activities against methicillin-resistant staphylococci and thus may prove to be useful in the treatment of infections caused by these multidrug-resistant organisms.
Figures
References
-
- Bignardi, G. E., N. Woodford, A. Chapman, A. P. Johnson, and D. C. E. Speller. 1996. Detection of the mec-A gene and phenotypic detection of resistance in Staphylococcus aureus isolates with borderline or low-level methicillin resistance. J. Antimicrob. Chemother. 37:53-63. - PubMed
-
- Chambers, H. F., M. Sachdeva, and S. Kennedy. 1990. Binding affinity for penicillin-binding protein 2a correlates with in vivo activity of β-lactam antibiotics against methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 162:705-710. - PubMed
-
- Dyke, K. G. H. 1966. Penicillinase production and intrinsic resistance to penicillins in Staphylococcus aureus. Lancet i:835-838. - PubMed
-
- Gradelski, E., E. Huczko, R. E. Kessler, D. P. Bonner, and J. Fung-Tomc. 1993. β-Lactamase parameters of heterogeneously and homogeneously methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 31:441-442. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous