Hemofiltrate CC chemokine 1[9-74] causes effective internalization of CCR5 and is a potent inhibitor of R5-tropic human immunodeficiency virus type 1 strains in primary T cells and macrophages
- PMID: 11897579
- PMCID: PMC127102
- DOI: 10.1128/AAC.46.4.982-990.2002
Hemofiltrate CC chemokine 1[9-74] causes effective internalization of CCR5 and is a potent inhibitor of R5-tropic human immunodeficiency virus type 1 strains in primary T cells and macrophages
Abstract
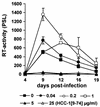
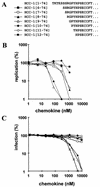
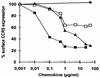
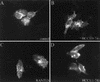
Proteolytic processing of the abundant plasmatic human CC chemokine 1 (HCC-1) generates a truncated form, HCC-1[9-74], which is a potent agonist of CCR1, CCR3, and CCR5; promotes calcium influx and chemotaxis of T lymphoblasts, monocytes, and eosinophils; and inhibits infection by CCR5-tropic human immunodeficiency virus type 1 (HIV-1) isolates. In the present study we demonstrate that HCC-1[9-74] interacts with the second external loop of CCR5 and inhibits replication of CCR5-tropic HIV-1 strains in both primary T cells and monocyte-derived macrophages. Low concentrations of the chemokine, however, frequently enhanced the replication of CCR5-tropic HIV-1 isolates but not the replication of X4-tropic HIV-1 isolates. Only HCC-1[9-74] and HCC-1[10-74], but not other HCC-1 length variants, displayed potent anti-HIV-1 activities. Fluorescence-activated cell sorter analysis revealed that HCC-1[9-74] caused up to 75% down-regulation of CCR5 cell surface expression, whereas RANTES (regulated on activation, normal T-cell expressed and secreted) achieved a reduction of only about 40%. Studies performed with green fluorescent protein-tagged CCR5 confirmed that both HCC-1[9-74] and RANTES, but not full-length HCC-1, mediated specific internalization of the CCR5 HIV-1 entry cofactor. Our results demonstrate that the interaction with HCC-1[9-74] causes effective intracellular sequestration of CCR5, but they also indicate that the effect of HCC-1[9-74] on viral replication is subject to marked cell donor- and HIV-1 isolate-dependent variations.
Figures
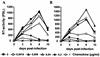



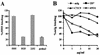
Similar articles
-
Agonist-induced internalization of CC chemokine receptor 5 as a mechanism to inhibit HIV replication.J Pharmacol Exp Ther. 2011 Jun;337(3):655-62. doi: 10.1124/jpet.111.179622. Epub 2011 Mar 9. J Pharmacol Exp Ther. 2011. PMID: 21389095
-
Genetic subtype-independent inhibition of human immunodeficiency virus type 1 replication by CC and CXC chemokines.J Virol. 1998 Jan;72(1):396-404. doi: 10.1128/JVI.72.1.396-404.1998. J Virol. 1998. PMID: 9420238 Free PMC article.
-
Inhibition of dual/mixed tropic HIV-1 isolates by CCR5-inhibitors in primary lymphocytes and macrophages.PLoS One. 2013 Jul 9;8(7):e68076. doi: 10.1371/journal.pone.0068076. Print 2013. PLoS One. 2013. PMID: 23874501 Free PMC article.
-
HIV co-receptors as targets for antiviral therapy.Curr Top Med Chem. 2004;4(9):883-93. doi: 10.2174/1568026043388501. Curr Top Med Chem. 2004. PMID: 15134547 Review.
-
Rational design of novel HIV-1 entry inhibitors by RANTES engineering.Vaccine. 2008 Jun 6;26(24):3008-15. doi: 10.1016/j.vaccine.2007.12.023. Epub 2008 Jan 10. Vaccine. 2008. PMID: 18243436 Free PMC article. Review.
Cited by
-
Cytokines Elevated in HIV Elite Controllers Reduce HIV Replication In Vitro and Modulate HIV Restriction Factor Expression.J Virol. 2017 Feb 28;91(6):e02051-16. doi: 10.1128/JVI.02051-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28053103 Free PMC article.
-
An initial investigation into endothelial CC chemokine expression in the human rheumatoid synovium.Cytokine. 2017 Sep;97:133-140. doi: 10.1016/j.cyto.2017.05.023. Cytokine. 2017. PMID: 28648867 Free PMC article.
-
CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of the Ligands of Receptors CCR1, CCR2, CCR3, and CCR4.Int J Mol Sci. 2020 Nov 9;21(21):8412. doi: 10.3390/ijms21218412. Int J Mol Sci. 2020. PMID: 33182504 Free PMC article. Review.
-
Respiratory ß-2-Microglobulin exerts pH dependent antimicrobial activity.Virulence. 2020 Dec;11(1):1402-1414. doi: 10.1080/21505594.2020.1831367. Virulence. 2020. PMID: 33092477 Free PMC article.
-
Nef-mediated enhancement of virion infectivity and stimulation of viral replication are fundamental properties of primate lentiviruses.J Virol. 2007 Dec;81(24):13852-64. doi: 10.1128/JVI.00904-07. Epub 2007 Oct 10. J Virol. 2007. PMID: 17928336 Free PMC article.
References
-
- Amara, A., S. Legall, O. Schwartz, J. Salamero, M. Montes, P. Loetscher, M. Baggiolini, J. L. Virelizier, and F. Arenza-Naseisdedos. 1997. HIV coreceptor down-regulation as antiviral principle: SDF-1α-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J. Exp. Med. 186:139-146. - PMC - PubMed
-
- Amzazi, S., L. Ylisastigui, Y. Bakri, L. Rabehi, L. Gattegno, M. Parmentier, J. C. Gluckman, and A. Benjouad. 1998. The inhibitory effect of RANTES on the infection of primary macrophages by R5 human immunodeficiency virus type-1 depends on the macrophage activation state. Virology 252:96-105. - PubMed
-
- Arenzana-Seisdedos, F., J. L. Virelizier, D. Rousset, I. Clark-Lewis, P. Loetscher, B. Moser, and M. Baggiolini. 1996. HIV blocked by chemokine antagonist. Nature 383:400.. - PubMed
-
- Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

