Oral administration of cyclopentane neuraminidase inhibitors protects ferrets against influenza virus infection
- PMID: 11897581
- PMCID: PMC127099
- DOI: 10.1128/AAC.46.4.996-1004.2002
Oral administration of cyclopentane neuraminidase inhibitors protects ferrets against influenza virus infection
Abstract
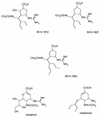
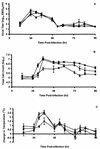
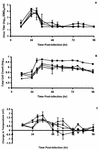
Several cyclopentane inhibitors of influenza virus neuraminidase that have inhibitory activities in tissue culture similar to those of zanamivir and oseltamivir have recently been described. These new inhibitors have been examined for efficacy against a virulent H3N2 influenza virus when administered orally to infected ferrets. Preliminary studies indicated that oral administration of BCX-1923, BCX-1827, or BCX-1812 (RWJ-270201) at a dose of 5 or 25 mg/kg of body weight was active in ferrets in reducing respiratory and constitutional signs and symptoms, but these antivirals affected virus titers in the upper and lower respiratory tracts only marginally. Of the three compounds, BCX-1812 seemed to be the most efficacious and was examined further at higher doses of 30 and 100 mg/kg. These doses significantly reduced peak virus titers in nasal washes and total virus shedding as measured by areas under the curve. Virus titers in lung homogenates were also reduced compared to those in controls, but the difference was not statistically significant. As was observed with BCX-1812 at lower doses, the nasal inflammatory cellular response, fever, and nasal signs were reduced while ferret activity was not, with activity remaining similar to uninfected animals.
Figures



Similar articles
-
Oral administration of a prodrug of the influenza virus neuraminidase inhibitor GS 4071 protects mice and ferrets against influenza infection.Antimicrob Agents Chemother. 1998 Mar;42(3):640-6. doi: 10.1128/AAC.42.3.640. Antimicrob Agents Chemother. 1998. PMID: 9517945 Free PMC article.
-
Comparison of the anti-influenza virus activity of RWJ-270201 with those of oseltamivir and zanamivir.Antimicrob Agents Chemother. 2001 Apr;45(4):1162-7. doi: 10.1128/AAC.45.4.1162-1167.2001. Antimicrob Agents Chemother. 2001. PMID: 11257030 Free PMC article.
-
In vivo influenza virus-inhibitory effects of the cyclopentane neuraminidase inhibitor RJW-270201.Antimicrob Agents Chemother. 2001 Mar;45(3):749-57. doi: 10.1128/AAC.45.3.749-757.2001. Antimicrob Agents Chemother. 2001. PMID: 11181355 Free PMC article.
-
Peramivir (BCX-1812, RWJ-270201): potential new therapy for influenza.Expert Opin Investig Drugs. 2002 Jun;11(6):859-69. doi: 10.1517/13543784.11.6.859. Expert Opin Investig Drugs. 2002. PMID: 12036429 Review.
-
RWJ-270201 (BCX-1812): a novel neuraminidase inhibitor for influenza.Philos Trans R Soc Lond B Biol Sci. 2001 Dec 29;356(1416):1905-13. doi: 10.1098/rstb.2001.1004. Philos Trans R Soc Lond B Biol Sci. 2001. PMID: 11779391 Free PMC article. Review.
Cited by
-
Phenotypic drug susceptibility assay for influenza virus neuraminidase inhibitors.Clin Diagn Lab Immunol. 2004 Jan;11(1):21-8. doi: 10.1128/cdli.11.1.21-28.2004. Clin Diagn Lab Immunol. 2004. PMID: 14715540 Free PMC article.
-
PEPT1-mediated prodrug strategy for oral delivery of peramivir.Asian J Pharm Sci. 2018 Nov;13(6):555-565. doi: 10.1016/j.ajps.2018.05.008. Epub 2018 Oct 3. Asian J Pharm Sci. 2018. PMID: 32104429 Free PMC article.
-
Subclinical brain injury caused by H5N1 influenza virus infection.J Virol. 2011 May;85(10):5202-7. doi: 10.1128/JVI.00239-11. Epub 2011 Mar 9. J Virol. 2011. PMID: 21389133 Free PMC article.
-
Susceptibilities of antiviral-resistant influenza viruses to novel neuraminidase inhibitors.Antimicrob Agents Chemother. 2005 Nov;49(11):4515-20. doi: 10.1128/AAC.49.11.4515-4520.2005. Antimicrob Agents Chemother. 2005. PMID: 16251290 Free PMC article.
-
Efficacy of oseltamivir therapy in ferrets inoculated with different clades of H5N1 influenza virus.Antimicrob Agents Chemother. 2007 Apr;51(4):1414-24. doi: 10.1128/AAC.01312-06. Epub 2007 Feb 12. Antimicrob Agents Chemother. 2007. PMID: 17296744 Free PMC article.
References
-
- Appleyard, G., and H. B. Maber. 1974. Plaque formation by influenza viruses in the presence of trypsin. J. Gen. Virol. 25:351-357. - PubMed
-
- Atigadda, V. R., W. J. Brouillette, F. Duarte, S. M. Ali, Y. S. Babu, S. Bantia, P. Chand, N. Chu, J. A. Montgomery, D. A. Walsh, E. A. Sudbeck, J. Finley, M. Luo, G. M. Air, and G. W. Laver. 1999. Potent inhibition of influenza sialidase by a benzoic acid containing a 2-pyrrolidinone substituent. J. Med. Chem. 42:2332-2343. - PubMed
-
- Babu, Y. S., P. Chand, S. Bantia, P. L. Kotian, A. Dehghani, Y. El-Kattan, T. Lin, T. L. Hutchinson, A. J. Elliott, C. D. Parker, S. L. Ananth, L. L. Horn, G. W. Laver, and J. A. Montgomery. 2000. BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J. Med. Chem. 43:3482-3486. - PubMed
-
- Bantia, S., C. D. Parker, S. L. Ananth, L. L. Horn, K. Andries, P. Chand, P. L. Kotian, A. Dehghani, Y. El-Kattan, T. Lin, T. L. Hutchinson, J. A. Montgomery, D. L. Kellog, and Y. S. Babu. 2001. Comparison of anti-influenza virus activity of RWJ-270201 with those of oseltamivir and zanamivir. Antimicrob. Agents Chemother. 45:1162-1167. - PMC - PubMed
-
- Barnett, J. M., A. Cadman, D. Gor, M. Dempsey, M. Walters, A. Candlin, M. Tisdale, P. J. Morley, I. J. Owens, R. J. Fenton, A. P. Lewis, E. C. J. Claas, G. F. Rimmelzwaan, R. De Groot, and A. D. M. E. Osterhaus. 2000. Zanamivir susceptibility monitoring and characterization of influenza virus clinical isolates obtained during phase II clinical efficacy studies. Antimicrob. Agents Chemother. 44:78-87. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

