In vitro low-level resistance to azoles in Candida albicans is associated with changes in membrane lipid fluidity and asymmetry
- PMID: 11897588
- PMCID: PMC127087
- DOI: 10.1128/AAC.46.4.1046-1052.2002
In vitro low-level resistance to azoles in Candida albicans is associated with changes in membrane lipid fluidity and asymmetry
Abstract
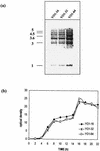
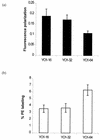
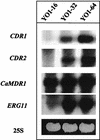
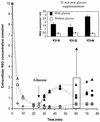
The present study tracks the development of low-level azole resistance in in vitro fluconazole-adapted strains of Candida albicans, which were obtained by serially passaging a fluconazole-susceptible dose-dependent strain, YO1-16 (fluconazole MIC, 16 microg ml(-1)) in increasing concentrations of fluconazole, resulting in strains YO1-32 (fluconazole MIC, 32 microg ml(-1)) and YO1-64 (MIC, 64 microg ml(-1)). We show that acquired resistance to fluconazole in this series of isolates is not a random process but is a gradually evolved complex phenomenon that involves multiple changes, which included the overexpression of ABC transporter genes, e.g., CDR1 and CDR2, and the azole target enzyme, ERG11. The sequential rise in fluconazole MICs in these isolates was also accompanied by cross-resistance to other azoles and terbinafine. Interestingly, fluorescent polarization measurements performed by using the fluorescent probe 1,6-diphenyl-1,3,5-hexatriene revealed that there was a gradual increase in membrane fluidity of adapted strains. The increase in fluidity was reflected by observed change in membrane order, which was considerably decreased (decrease in fluorescence polarization values, P value) in the adapted strain (P value of 0.1 in YO1-64, compared to 0.19 in the YO1-16 strain). The phospholipid composition of the adapted strain was not significantly altered; however, ergosterol content was reduced in YO1-64 from that in the YO1-16 strain. The asymmetrical distribution of phosphatidylethanolamine (PE) between two monolayers of plasma membrane was also changed, with PE becoming more exposed to the outer monolayer in the YO1-64 strain. The results of the present study suggest for the first time that changes in the status of membrane lipid phase and asymmetry could contribute to azole resistance in C. albicans.
Figures

Similar articles
-
Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters.Antimicrob Agents Chemother. 1995 Nov;39(11):2378-86. doi: 10.1128/AAC.39.11.2378. Antimicrob Agents Chemother. 1995. PMID: 8585712 Free PMC article.
-
Membrane fluidity and lipid composition in clinical isolates of Candida albicans isolated from AIDS/HIV patients.Acta Microbiol Immunol Hung. 2007 Dec;54(4):367-77. doi: 10.1556/AMicr.54.2007.4.4. Acta Microbiol Immunol Hung. 2007. PMID: 18088010
-
Effects of azole treatments on the physical properties of Candida albicans plasma membrane: a spin probe EPR study.Biochim Biophys Acta. 2014 Jan;1838(1 Pt B):465-73. doi: 10.1016/j.bbamem.2013.10.015. Epub 2013 Oct 31. Biochim Biophys Acta. 2014. PMID: 24184423
-
Antifungal drug resistance in pathogenic fungi.Med Mycol. 1998;36 Suppl 1:119-28. Med Mycol. 1998. PMID: 9988500 Review.
-
The genetic basis of fluconazole resistance development in Candida albicans.Biochim Biophys Acta. 2002 Jul 18;1587(2-3):240-8. doi: 10.1016/s0925-4439(02)00087-x. Biochim Biophys Acta. 2002. PMID: 12084466 Review.
Cited by
-
Functional analysis of CaIPT1, a sphingolipid biosynthetic gene involved in multidrug resistance and morphogenesis of Candida albicans.Antimicrob Agents Chemother. 2005 Aug;49(8):3442-52. doi: 10.1128/AAC.49.8.3442-3452.2005. Antimicrob Agents Chemother. 2005. PMID: 16048959 Free PMC article.
-
Synergistic effect of calcineurin inhibitors and fluconazole against Candida albicans biofilms.Antimicrob Agents Chemother. 2008 Mar;52(3):1127-32. doi: 10.1128/AAC.01397-07. Epub 2008 Jan 7. Antimicrob Agents Chemother. 2008. PMID: 18180354 Free PMC article.
-
Physiological adventures in Candida albicans: farnesol and ubiquinones.Microbiol Mol Biol Rev. 2024 Mar 27;88(1):e0008122. doi: 10.1128/mmbr.00081-22. Epub 2024 Mar 4. Microbiol Mol Biol Rev. 2024. PMID: 38436263 Free PMC article. Review.
-
Deletion of RAP1 affects iron homeostasis, azole resistance, and virulence in Candida albicans.mSphere. 2025 May 27;10(5):e0015525. doi: 10.1128/msphere.00155-25. Epub 2025 Apr 23. mSphere. 2025. PMID: 40265929 Free PMC article.
-
Novel Regulatory Mechanisms of Pathogenicity and Virulence to Combat MDR in Candida albicans.Int J Microbiol. 2013;2013:240209. doi: 10.1155/2013/240209. Epub 2013 Sep 16. Int J Microbiol. 2013. PMID: 24163696 Free PMC article. Review.
References
-
- Ansari, S., P. Gupta, S. K. Mahanty, and R. Prasad. 1993. The uptake of amino acids by erg mutants of Candida albicans. J. Med. Vet. Mycol. 31:377-386.
-
- Bayer, A. S., R. Prasad, J. Chandra, A. Koul, M. Smriti, A. Varma, R. A. Skurray, N. Firth, M. H. Brown, S. Koo, and M. R. Yeaman. 2000. In vitro resistance of Staphylococcus aureus to thrombin-induced platelet microbicidal protein is associated with alterations in cytoplasmic membrane fluidity. Infect. Immun. 68:3548-3553. - PMC - PubMed
-
- Bolard, J., and J. Milhaud. 1996. Interaction of anti-Candida amphotericin B (and other polyene antibiotics) with lipids, p. 253-274. In R. Prasad and M. A. Ghannoum (ed.), Lipids of pathogenic fungi. CRC Press, Boca Raton, Fla.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

