In vitro antiplasmodium effects of dermaseptin S4 derivatives
- PMID: 11897590
- PMCID: PMC127115
- DOI: 10.1128/AAC.46.4.1059-1066.2002
In vitro antiplasmodium effects of dermaseptin S4 derivatives
Abstract
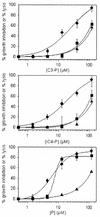
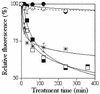
The 13-residue dermaseptin S4 derivative K(4)S4(1-13)a (P) was previously shown to kill intraerythrocytic malaria parasites through the lysis of the host cells. In this study, we have sought peptides that will kill the parasite without lysing the erythrocyte. To produce such peptides, 26 compounds of variable structure and size were attached to the N terminus of P and screened for antiplasmodium and hemolytic activities in cultures of Plasmodium falciparum. Results from this screen indicated that increased hydrophobicity results in amplified antiplasmodium effect, irrespective of the linearity or bulkiness of the additive. However, increased hydrophobicity also was generally associated with increased hemolysis, with the exception of two derivatives: propionyl-P (C3-P) and isobutyryl-P (iC4-P). Both acyl-peptides were more effective than P, with 50% growth inhibition at 3.8, 4.3, and 7.7 microM, respectively. The antiparasitic effect was time dependent and totally irreversible, implying a cytotoxic effect. The peptides were also investigated in parallel for their ability to inhibit parasite growth and to induce hemolysis in infected and uninfected erythrocytes. Whereas the dose dependence of growth inhibition and hemolysis of infected cells overlapped when cells were treated with P, the acyl-peptides exerted 50% growth inhibition at concentrations that did not cause hemolysis. Noticeably, the acyl derivatives, but not P, were able to dissipate the parasite plasma membrane potential and cause depletion of intraparasite potassium under nonhemolytic conditions. These results clearly demonstrate that the acyl-peptides can affect parasite viability in a manner that is dissociated from lysis of the host cell. Overall, the data indicate the potential usefulness of this strategy for development of selective peptides as investigative tools and eventually as antimalarial agents.
Figures


Similar articles
-
Direct interaction of dermaseptin S4 aminoheptanoyl derivative with intraerythrocytic malaria parasite leading to increased specific antiparasitic activity in culture.J Biol Chem. 2002 Jul 5;277(27):24067-72. doi: 10.1074/jbc.M202089200. Epub 2002 Apr 5. J Biol Chem. 2002. PMID: 11937508
-
Antimalarial activities of dermaseptin S4 derivatives.Antimicrob Agents Chemother. 2000 Sep;44(9):2442-51. doi: 10.1128/AAC.44.9.2442-2451.2000. Antimicrob Agents Chemother. 2000. PMID: 10952593 Free PMC article.
-
Selective cytotoxicity of dermaseptin S3 toward intraerythrocytic Plasmodium falciparum and the underlying molecular basis.J Biol Chem. 1997 Dec 12;272(50):31609-16. doi: 10.1074/jbc.272.50.31609. J Biol Chem. 1997. PMID: 9395500
-
Antibacterial properties of dermaseptin S4 derivatives with in vivo activity.Antimicrob Agents Chemother. 2002 Mar;46(3):689-94. doi: 10.1128/AAC.46.3.689-694.2002. Antimicrob Agents Chemother. 2002. PMID: 11850249 Free PMC article.
-
Structure-activity relationship study of antimicrobial dermaseptin S4 showing the consequences of peptide oligomerization on selective cytotoxicity.J Biol Chem. 2000 Feb 11;275(6):4230-8. doi: 10.1074/jbc.275.6.4230. J Biol Chem. 2000. PMID: 10660589
Cited by
-
A chimeric peptide composed of a dermaseptin derivative and an RNA III-inhibiting peptide prevents graft-associated infections by antibiotic-resistant staphylococci.Antimicrob Agents Chemother. 2004 Jul;48(7):2544-50. doi: 10.1128/AAC.48.7.2544-2550.2004. Antimicrob Agents Chemother. 2004. PMID: 15215107 Free PMC article.
-
Antibacterial properties of dermaseptin S4 derivatives under extreme incubation conditions.Antimicrob Agents Chemother. 2006 Feb;50(2):490-7. doi: 10.1128/AAC.50.2.490-497.2006. Antimicrob Agents Chemother. 2006. PMID: 16436701 Free PMC article.
-
Bioactive Peptides: Synthesis, Properties, and Applications in the Packaging and Preservation of Food.Compr Rev Food Sci Food Saf. 2012 Mar;11(2):187-204. doi: 10.1111/j.1541-4337.2011.00179.x. Epub 2012 Feb 29. Compr Rev Food Sci Food Saf. 2012. PMID: 32368201 Free PMC article.
-
Computational reverse-engineering of a spider-venom derived peptide active against Plasmodium falciparum SUB1.PLoS One. 2011;6(7):e21812. doi: 10.1371/journal.pone.0021812. Epub 2011 Jul 27. PLoS One. 2011. PMID: 21818266 Free PMC article.
-
Antiplasmodial activity of lauryl-lysine oligomers.Antimicrob Agents Chemother. 2007 May;51(5):1753-9. doi: 10.1128/AAC.01288-06. Epub 2007 Feb 16. Antimicrob Agents Chemother. 2007. PMID: 17307975 Free PMC article.
References
-
- Andreu, D., and L. Rivas. 1998. Animal antimicrobial peptides: an overview. Biopolymers 47:415-433. - PubMed
-
- Blondelle, E. S., and K. Lohner. 2000. Combinatorial libraries: a tool to design antimicrobial and antifungal peptide analogues having lytic specificities for structure-activity relationship studies. Biopolymers 55:74-87. - PubMed
-
- Boman, H. G. 1995. Peptide antibiotics and their role in innate immunity. Annu. Rev. Immunol. 13:61-92. - PubMed
-
- Chen, J., T. J. Falla, H. Liu, M. A. Hurst, C. A. Fujii, D. A. Mosca, J. R. Embree, D. J. Loury, P. A. Radel, C. C. Chang, L. Gu, and J. C. Fiddes. 2000. Development of protegrins for the treatment and prevention of oral mucositis: structure activity relationships of synthetic protegrin analogues. Biopolymers 55:88-98. - PubMed
-
- Feder, R., A. Dagan, and A. Mor. 2000. SAR study of antimicrobial dermaseptin S4 showing the consequences of peptide oligomerization on selective cytotoxicity. J. Biol. Chem. 275:4230-4238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

