Alpha(1H) mRNA in single skeletal muscle fibres accounts for T-type calcium current transient expression during fetal development in mice
- PMID: 11897840
- PMCID: PMC2290181
- DOI: 10.1113/jphysiol.2001.013246
Alpha(1H) mRNA in single skeletal muscle fibres accounts for T-type calcium current transient expression during fetal development in mice
Abstract
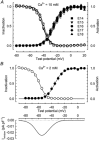
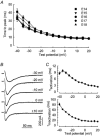
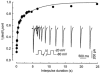
Calcium channels are essential for excitation-contraction coupling and muscle development. At the end of fetal life, two types of Ca(2+) currents can be recorded in muscle cells. Whereas L-type Ca(2+) channels have been extensively studied, T-type channels have been poorly characterized in skeletal muscle. We describe here the functional and molecular properties of T-type calcium channels in developing mouse skeletal muscle. The T-type current density increased transiently during prenatal myogenesis with a maximum at embryonic day E16 followed by a drastic decrease until birth. This current showed similar electrophysiological and pharmacological properties at all examined stages. It displayed a wide window current centred at about -35 and -55 mV in 10 and 2 mM external Ca(2+), respectively. Activation and inactivation kinetics were fast (3 and 16 ms, respectively). The current was inhibited by nickel and amiloride with an IC(50) of 5.4 and 156 microM, respectively, values similar to those described for cloned T-type alpha(1H) channels. Whole muscle tissue RT-PCR analysis revealed mRNAs corresponding to alpha(1H) and alpha(1G) subunits in the fetus but not in the adult. However, single-fibre RT-PCR demonstrated that only alpha(1H) mRNA was present in prenatal fibres, suggesting that the alpha(1G) transcript present in muscle tissue must be expressed by non-skeletal muscle cells. Altogether, these results demonstrate that the alpha(1H) subunit generates functional T-type calcium channels in developing skeletal muscle fibres and suggest that these channels are involved in the early stages of muscle differentiation.
Figures



References
-
- Bijlenga P, Liu J-H, Espinos E, Haenggli C-A, Fischer-Lougheed J, Bader CR, Bernheim L. T-type alpha1H Ca2+ channels are involved in Ca2+ signaling during terminal differentiation (fusion) of human myoblasts. Proceedings of the National Academy of Sciences of the USA. 2000;97:7627–7632. - PMC - PubMed
-
- Bossu J-L, Feltz A, Thomann JM. Depolarization elicits two distinct calcium currents in vertebrate sensory neurones. Pflügers Archiv. 1985;403:360–368. - PubMed
-
- Carbone E, Lux HD. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984;310:501–502. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

