Differential effects of sarcoplasmic reticular Ca(2+)-ATPase inhibition on charge movements and calcium transients in intact amphibian skeletal muscle fibres
- PMID: 11897856
- PMCID: PMC2290190
- DOI: 10.1113/jphysiol.2001.013095
Differential effects of sarcoplasmic reticular Ca(2+)-ATPase inhibition on charge movements and calcium transients in intact amphibian skeletal muscle fibres
Abstract
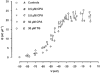
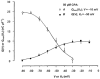
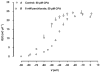
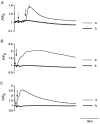
A hypothesis in which intramembrane charge reflects a voltage sensing process allosterically coupled to transitions in ryanodine receptor (RyR)-Ca(2+) release channels as opposed to one driven by release of intracellularly stored Ca(2+) would predict that such charging phenomena should persist in skeletal muscle fibres unable to release stored Ca(2+). Charge movement components were accordingly investigated in intact voltage-clamped amphibian fibres treated with known sarcoplasmic reticular (SR) Ca(2+)-ATPase inhibitors. Cyclopiazonic acid (CPA) pretreatment abolished Ca(2+) transients in fluo-3-loaded fibres following even prolonged applications of caffeine (10 mM) or K(+) (122 mM). Both CPA and thapsigargin (TG) transformed charge movements that included delayed (q(gamma)) "hump" components into simpler decays. However, steady-state charge-voltage characteristics were conserved to values (maximum charge, Q(max) approximately equal to 20-25 nC microF(-1); transition voltage, V* approximately equal to -40 to-50 mV; steepness factor, k approximately equal to 6-9 mV; holding voltage -90 mV) indicating persistent q(gamma) charge. The features of charge inactivation similarly suggested persistent q(beta) and q(gamma) charge contributions in CPA-treated fibres. Perchlorate (8.0 mM) restored the delayed kinetics shown by "on" q(gamma) charge movements, prolonged their "off" decays, conserved both Q(max) and k, yet failed to restore the capacity of such CPA-treated fibres for Ca(2+) release. Introduction of perchlorate (8.0 mM) or caffeine (0.2 mM) to tetracaine (2.0 mM)-treated fibres, also known to restore q(gamma) charge, similarly failed to restore Ca(2+) transients. Steady-state intramembrane q(gamma) charge thus persists with modified kinetics that can be restored to its normally complex waveform by perchlorate, even in intact muscle fibres unable to release Ca(2+). It is thus unlikely that q(gamma) charge movement is a consequence of SR Ca(2+) release rather than changes in tubular membrane potential.
Figures




Similar articles
-
Charge movements in intact amphibian skeletal muscle fibres in the presence of cardiac glycosides.J Physiol. 2001 Apr 15;532(Pt 2):509-23. doi: 10.1111/j.1469-7793.2001.0509f.x. J Physiol. 2001. PMID: 11306668 Free PMC article.
-
The influence of perchlorate ions on complex charging transients in amphibian striated muscle.J Physiol. 1998 Feb 1;506 ( Pt 3)(Pt 3):699-714. doi: 10.1111/j.1469-7793.1998.699bv.x. J Physiol. 1998. PMID: 9503332 Free PMC article.
-
The influence of caffeine on intramembrane charge movements in intact frog striated muscle.J Physiol. 1998 Nov 1;512 ( Pt 3)(Pt 3):707-21. doi: 10.1111/j.1469-7793.1998.707bd.x. J Physiol. 1998. PMID: 9769415 Free PMC article.
-
Kinetic isoforms of intramembrane charge in intact amphibian striated muscle.J Gen Physiol. 1996 Apr;107(4):515-34. doi: 10.1085/jgp.107.4.515. J Gen Physiol. 1996. PMID: 8722564 Free PMC article.
-
Dual actions of tetracaine on intramembrane charge in amphibian striated muscle.J Physiol. 1997 Jun 15;501 ( Pt 3)(Pt 3):589-606. doi: 10.1111/j.1469-7793.1997.589bm.x. J Physiol. 1997. PMID: 9218219 Free PMC article.
Cited by
-
Effects of sphingosine 1-phosphate on excitation-contraction coupling in mammalian skeletal muscle.J Muscle Res Cell Motil. 2003;24(8):539-54. doi: 10.1023/b:jure.0000009898.02325.58. J Muscle Res Cell Motil. 2003. PMID: 14870969
-
Reciprocal dihydropyridine and ryanodine receptor interactions in skeletal muscle activation.J Muscle Res Cell Motil. 2011 Nov;32(3):171-202. doi: 10.1007/s10974-011-9262-9. Epub 2011 Oct 13. J Muscle Res Cell Motil. 2011. PMID: 21993921 Review.
-
L-type Ca2+ channel and ryanodine receptor cross-talk in frog skeletal muscle.J Physiol. 2004 Feb 15;555(Pt 1):137-52. doi: 10.1113/jphysiol.2003.051730. Epub 2003 Dec 5. J Physiol. 2004. PMID: 14660705 Free PMC article.
-
FPL-64176 alters both charge movement and Ca2+ release properties in amphibian muscle fibres.Pflugers Arch. 2004 Mar;447(6):922-7. doi: 10.1007/s00424-003-1190-0. Pflugers Arch. 2004. PMID: 15061146
-
Association of the Igamma and Idelta charge movement with calcium release in frog skeletal muscle.Biophys J. 2005 Feb;88(2):1030-45. doi: 10.1529/biophysj.104.048215. Epub 2004 Nov 8. Biophys J. 2005. PMID: 15533918 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

