Excitability changes in human corticospinal projections to forearm muscles during voluntary movement of ipsilateral foot
- PMID: 11897859
- PMCID: PMC2290195
- DOI: 10.1113/jphysiol.2001.013282
Excitability changes in human corticospinal projections to forearm muscles during voluntary movement of ipsilateral foot
Abstract
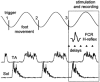
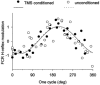
Excitability of the H-reflex in the relaxed flexor carpi radialis (FCR) muscle was tested during voluntary oscillations of the ipsilateral foot at five evenly spaced delays during a 600 ms cycle. In some experiments the H-reflex was conditioned by transcranial magnetic stimulation (TMS). With the hand prone, the amplitude of the FCR H-reflex was modulated sinusoidally with the same period as the foot oscillation, the modulation peak occurring in coincidence with contraction of the foot plantar-flexor soleus and the trough during contraction of the extensor tibialis anterior. When the H-reflex was facilitated by TMS at short latency (conditioning-test interval: -2 to -3.5 ms), the modulation was larger than that occurring with an unconditioned reflex of comparable size. This suggests that both the peripheral and the corticospinal components of the facilitated response were modulated in parallel. When the H-reflex was tested 40-60 ms after conditioning, i.e. during the cortical "silent period" induced by TMS, no direct effect was produced on the reflex size but the foot-associated modulation was deeply depressed. These results suggest that the reflex modulation may depend on activity fluctuations in the cortical motor area innervating the forearm motoneurones. It is proposed that when the foot is rhythmically oscillated, along with the full activation of the foot cortical area a simultaneous lesser co-activation of the forearm area produces a subliminal cyclic modulation of cervical motoneurones excitability. Should the two limbs be moved together, the time course of this modulation would favour isodirectional movements of the prone hand and foot, indeed the preferential coupling observed when hand and foot are voluntarily oscillated.
Figures




References
-
- Aruin AS, Forrest WR, Latash ML. Anticipatory postural adjustments in conditions of postural instability. Electroencephalography & Clinical Neurophysiology. 1998;109:350–359. - PubMed
-
- Aruin AS, Latash ML. Directional specificity of postural muscles in feed-forward postural reactions during fast voluntary arm movements. Experimental Brain Research. 1995;103:323–332. - PubMed
-
- Aruin AS, Latash ML. Anticipatory postural adjustments during self-initiated perturbations of different magnitude triggered by a standard motor action. Electroencephalography & Clinical Neurophysiology. 1996;101:497–503. - PubMed
-
- Baldissera F, Borroni P, Cavallari P. Neural compensation for mechanical differences between hand and foot during coupled oscillations of the two segments. Experimental Brain Research. 2000;133:165–177. - PubMed
-
- Baldissera F, Borroni P, Cavallari P, Cerri G. Cyclic modulation of the excitability of resting forearm muscles is related to cyclic contraction of foot movers, not to movement. Pflügers Archiv. 2001;442:R7(4).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

