Developmental changes in cerebral autoregulatory capacity in the fetal sheep parietal cortex
- PMID: 11897864
- PMCID: PMC2290182
- DOI: 10.1113/jphysiol.2001.012590
Developmental changes in cerebral autoregulatory capacity in the fetal sheep parietal cortex
Abstract
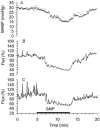
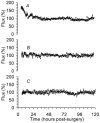
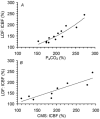
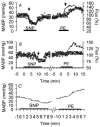
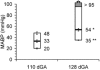
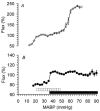
We validated laser Doppler flowmetry (LDF) for long-term monitoring and detection of acute changes of local cerebral blood flow (lCBF) in chronically instrumented fetal sheep. Using LDF, we estimated developmental changes of cerebral autoregulation. Single fibre laser probes (0.4 mm in diameter) were implanted in and surface probes were placed on the parietal cerebral cortex at 105 +/- 2 (n = 7) and 120 +/- 2 days gestational age (dGA, n = 7). Basal lCBF was monitored over 5 days followed by a hypercapnic challenge (fetal arterial partial pressure of CO(2), P(a,CO2): 83 +/- 3 mmHg) during which lCBF changes obtained by LDF were compared to those obtained with coloured microspheres (CMSs). Mean arterial blood pressure (MABP) was increased and decreased using phenylephrine and sodium nitroprusside at 110 +/- 2 and 128 +/- 2 dGA. Intracortical and cortical surface laser probes gave stable measurements over 5 days. The lCBF increase during hypercapnia obtained by LDF correlated well with flows obtained using CMS (r = 0.89, P < 0.01). The signals of intracortical and surface laser probes also correlated well (r = 0.91, P < 0.01). Gliosis of 0.35 +/- 0.06 mm around the tip of intracortical probes did not affect the measurements. The range of MABP over which cerebral autoregulation was observed increased from 20-48 mmHg at 110 dGA to 35 to > 95 mmHg at 128 dGA (P < 0.05). Since MABP increased from 33 to 54 mmHg over this period (P < 0.01), the range between the lower limit of cerebral autoregulation and the MABP increased from 13 mmHg at 110 dGA to 19 mmHg at 128 dGA (P < 0.01). LDF is a reliable tool to assess dynamic changes in cerebral perfusion continuously in fetal sheep.
Figures



Similar articles
-
Laser Doppler flowmetry is valid for measurement of cerebral blood flow autoregulation lower limit in rats.Exp Physiol. 2005 May;90(3):349-55. doi: 10.1113/expphysiol.2004.029512. Epub 2005 Jan 14. Exp Physiol. 2005. PMID: 15653714
-
Variability in the magnitude of the cerebral blood flow response and the shape of the cerebral blood flow-pressure autoregulation curve during hypotension in normal rats [corrected].Anesthesiology. 2002 Aug;97(2):488-96. doi: 10.1097/00000542-200208000-00028. Anesthesiology. 2002. PMID: 12151941
-
The effects of halothane and isoflurane on cerebrocortical microcirculation and autoregulation as assessed by laser-Doppler flowmetry.Anesth Analg. 1994 Jul;79(1):58-65. Anesth Analg. 1994. PMID: 8010455
-
Laser-Doppler flowmetry in neurosurgery.J Neurosurg Anesthesiol. 1993 Jul;5(3):151-8. doi: 10.1097/00008506-199307000-00004. J Neurosurg Anesthesiol. 1993. PMID: 8400753 Review.
-
[Mechanisms of cerebral autoregulation, assessment and interpretation by means of transcranial doppler sonography].Fortschr Neurol Psychiatr. 2000 Sep;68(9):398-412. doi: 10.1055/s-2000-11798. Fortschr Neurol Psychiatr. 2000. PMID: 11037638 Review. German.
Cited by
-
Cerebral Blood Flow Monitoring in High-Risk Fetal and Neonatal Populations.Front Pediatr. 2022 Jan 11;9:748345. doi: 10.3389/fped.2021.748345. eCollection 2021. Front Pediatr. 2022. PMID: 35087771 Free PMC article. Review.
-
Dopamine therapy does not affect cerebral autoregulation during hypotension in newborn piglets.PLoS One. 2017 Jan 31;12(1):e0170738. doi: 10.1371/journal.pone.0170738. eCollection 2017. PLoS One. 2017. PMID: 28141842 Free PMC article.
-
Development affects in vitro vascular tone and calcium sensitivity in ovine cerebral arteries.J Physiol. 2004 Aug 1;558(Pt 3):883-96. doi: 10.1113/jphysiol.2003.056945. Epub 2004 May 6. J Physiol. 2004. PMID: 15131239 Free PMC article.
-
Redistribution of Cerebral Blood Flow during Severe Hypovolemia and Reperfusion in a Sheep Model: Critical Role of α1-Adrenergic Signaling.Int J Mol Sci. 2017 May 11;18(5):1031. doi: 10.3390/ijms18051031. Int J Mol Sci. 2017. PMID: 28492488 Free PMC article.
-
Instrumentation of Near-term Fetal Sheep for Multivariate Chronic Non-anesthetized Recordings.J Vis Exp. 2015 Oct 25;(105):e52581. doi: 10.3791/52581. J Vis Exp. 2015. PMID: 26555084 Free PMC article.
References
-
- Anderson RE, Michenfelder JD, Sundt TM. Brain intracellular pH, blood flow, and blood-brain barrier differences with barbiturate and halothane anesthesia in the cat. Anesthesiology. 1980;52:201–206. - PubMed
-
- Armstead WM, Zuckerman SL, Shibata M, Parfenova H, Leffler CW. Different pial arteriolar responses to acetylcholine in the newborn and juvenile pig. Journal of Cerebral Blood Flow and Metabolism. 1994;14:1088–1095. - PubMed
-
- Ashwal S, Dale PS, Longo LD. Regional cerebral blood flow: studies in the fetal lamb during hypoxia, hypercapnia, acidosis, and hypotension. Pediatric Research. 1984;18:1309–1316. - PubMed
-
- Bazin JE. Effects of anesthetic agents on intracranial pressure. Annales Françaises d’ Anesthésie et de Réanimation. 1997;16:445–452. - PubMed
-
- Brace RA, Brittingham DS. Fetal vascular pressure and heart rate responses to nonlabor uterine contractions. American Journal of Physiology. 1986;251:R409–416. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

