The alpha(1D)-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction
- PMID: 11901185
- PMCID: PMC150908
- DOI: 10.1172/JCI14001
The alpha(1D)-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction
Abstract
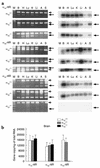
To investigate the physiological role of the alpha(1D)-adrenergic receptor (alpha(1D)-AR) subtype, we created mice lacking the alpha(1D)-AR (alpha(1D)(-/-)) by gene targeting and characterized their cardiovascular function. In alpha(1D)-/- mice, the RT-PCR did not detect any transcript of the alpha(1D)-AR in any tissue examined, and there was no apparent upregulation of other alpha(1)-AR subtypes. Radioligand binding studies showed that alpha(1)-AR binding capacity in the aorta was lost, while that in the heart was unaltered in alpha(1D)-/- mice. Non-anesthetized alpha(1D)-/- mice maintained significantly lower basal systolic and mean arterial blood pressure conditions, relative to wild-type mice, and they showed no significant change in heart rate or in cardiac function, as assessed by echocardiogram. Besides hypotension, the pressor responses to phenylephrine and norepinephrine were decreased by 30-40% in alpha(1D)-/- mice. Furthermore, the contractile response of the aorta and the pressor response of isolated perfused mesenteric arterial beds to alpha(1)-AR stimulation were markedly reduced in alpha(1D)-/- mice. We conclude that the alpha(1D)-AR participates directly in sympathetic regulation of systemic blood pressure by vasoconstriction.
Figures

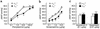

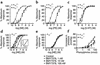
References
-
- Hoffman, B.B., and Lefkowitz, R.J. 1996. Catecholamines, sympathomimetic drugs, and adrenergic receptor antagonists. In The pharmacological basis of the therapeutics. J.G. Hardman, E. Limbird, L.P.B. Molinoff, and R.W. Ruddon, editors. McGraw-Hill. New York, New York, USA. 199–248.
-
- Schwinn DA, et al. Molecular cloning and expression of the cDNA for a novel α1-adrenergic receptor subtype. J Biol Chem. 1990;265:8183–8189. - PubMed
-
- Perez DM, Piascik MT, Graham RM. Solution-phase library screening for the identification of rare clones: isolation of an α1D-adrenergic receptor cDNA. Mol Pharmacol. 1991;40:876–883. - PubMed
-
- Hirasawa A, et al. Cloning, functional expression and tissue distribution of human cDNA for the α1C-adrenergic receptor. Biochem Biophys Res Commun. 1993;195:902–909. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

