Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite
- PMID: 11901190
- PMCID: PMC150913
- DOI: 10.1172/JCI14442
Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite
Abstract
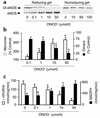
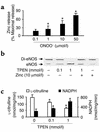
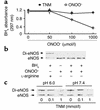
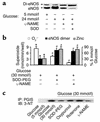
Nitric oxide (NO) is produced by NO synthase (NOS) in many cells and plays important roles in the neuronal, muscular, cardiovascular, and immune systems. In various disease conditions, all three types of NOS (neuronal, inducible, and endothelial) are reported to generate oxidants through unknown mechanisms. We present here the first evidence that peroxynitrite (ONOO(-)) releases zinc from the zinc-thiolate cluster of endothelial NOS (eNOS) and presumably forms disulfide bonds between the monomers. As a result, disruption of the otherwise SDS-resistant eNOS dimers occurs under reducing conditions. eNOS catalytic activity is exquisitely sensitive to ONOO(-), which decreases NO synthesis and increases superoxide anion (O(2)(.-)) production by the enzyme. The reducing cofactor tetrahydrobiopterin is not oxidized, nor does it prevent oxidation of eNOS by the same low concentrations of OONO(-). Furthermore, eNOS derived from endothelial cells exposed to elevated glucose produces more O(2)(.-), and, like eNOS purified from diabetic LDL receptor-deficient mice, contains less zinc and fewer SDS-resistant dimers. Hence, eNOS exposure to oxidants including ONOO(-) causes increased enzymatic uncoupling and generation of O(2)(.-) in diabetes, contributing further to endothelial cell oxidant stress. Regulation of the zinc-thiolate center of NOS by ONOO(-) provides a novel mechanism for modulation of the enzyme function in disease.
Figures

References
-
- Marletta MA. Nitric oxide synthase. Structure and mechanism. J Biol Chem. 1993;268:12231–12234. - PubMed
-
- Nathan C, Xie QW. Regulation of biosynthesis of nitric oxide. J Biol Chem. 1994;269:13725–13728. - PubMed
-
- Raman CS, et al. Crystal structure of constitutive endothelial nitric oxide synthase: a paradigm for pterin function involving a novel metal center. Cell. 1998;95:939–950. - PubMed
-
- Ludwig ML, Marletta MA. A new decoration for nitric oxide synthase - a Zn(Cys)4 site. Structure Fold Des. 1999;7:R73–R79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

