Transient receptor potential 1 regulates capacitative Ca(2+) entry and Ca(2+) release from endoplasmic reticulum in B lymphocytes
- PMID: 11901194
- PMCID: PMC2193746
- DOI: 10.1084/jem.20011758
Transient receptor potential 1 regulates capacitative Ca(2+) entry and Ca(2+) release from endoplasmic reticulum in B lymphocytes
Abstract
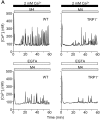
Capacitative Ca(2+) entry (CCE) activated by release/depletion of Ca(2+) from internal stores represents a major Ca(2+) influx mechanism in lymphocytes and other nonexcitable cells. Despite the importance of CCE in antigen-mediated lymphocyte activation, molecular components constituting this mechanism remain elusive. Here we demonstrate that genetic disruption of transient receptor potential (TRP)1 significantly attenuates both Ca(2+) release-activated Ca(2+) currents and inositol 1,4,5-trisphosphate (IP(3))-mediated Ca(2+) release from endoplasmic reticulum (ER) in DT40 B cells. As a consequence, B cell antigen receptor-mediated Ca(2+) oscillations and NF-AT activation are reduced in TRP1-deficient cells. Thus, our results suggest that CCE channels, whose formation involves TRP1 as an important component, modulate IP(3) receptor function, thereby enhancing functional coupling between the ER and plasma membrane in transduction of intracellular Ca(2+) signaling in B lymphocytes.
Figures

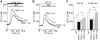

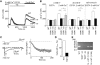

References
-
- Clapham, D.E. 1995. Calcium signaling. Cell. 80:259–268. - PubMed
-
- Berridge, M.J., M.D. Bootman, and P. Lipp. 1998. Calcium-a life and death signal. Nature. 395:645–648. - PubMed
-
- Putney, J.W., and G.S.J. Bird. 1993. The signal for capacitative calcium entry. Cell. 75:199–201. - PubMed
-
- Dolmetsch, R.E., R.S. Lewis, C.C. Goodnow, and J.I. Healy. 1997. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 386:855–858. - PubMed
-
- Li, W.-H., J. Llopis, M. Whitney, G. Zlokarnik, and R.Y. Tsien. 1998. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature. 392:936–941. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

