Major histocompatibility complex class II presentation of cell-associated antigen is mediated by CD8alpha+ dendritic cells in vivo
- PMID: 11901195
- PMCID: PMC2193737
- DOI: 10.1084/jem.20010898
Major histocompatibility complex class II presentation of cell-associated antigen is mediated by CD8alpha+ dendritic cells in vivo
Abstract
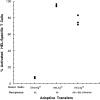
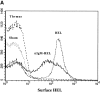
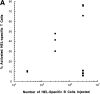
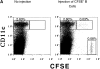
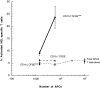
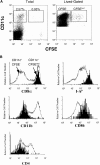
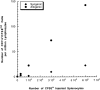
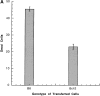
Antigen-specific B cells express major histocompatibility complex class II and can present antigen directly to T cells. Adoptive transfer experiments using transgenic B and T cells demonstrated that antigen-specific B cells can also efficiently transfer antigen to another cell for presentation to T cells in vivo. To identify the antigen-presenting cell that receives antigens from B cells, a strategy was developed to follow the traffic of B cell-derived proteins in vivo. B cells were labeled with the fluorescent dye CFSE and loaded with antigen, before adoptive transfer into recipient mice. Populations of splenocytes from the recipient mice were later assayed for the presence of fluorescent proteins and for the ability to activate T cells. A small number of CD8alpha+CD4-CD11b(lo) dendritic cells (DCs) contain proteins transferred from B cells and these DCs effectively present antigens derived from the B cells to T cells. The results suggest that CD8alpha+ DCs sample the cells and membranes in their environment for presentation to T cells circulating through the T cell zone. This function of CD8alpha+ DCs may be relevant to the priming of an immune response or the induction of T cell tolerance.
Figures








References
-
- Bennett, S.R., F.R. Carbone, F. Karamalis, R.A. Flavell, J.F. Miller, and W.R. Heath. 1998. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 393:478–480. - PubMed
-
- Batista, F.D., D. Iber, and M.S. Neuberger. 2001. B cells acquire antigen from target cells after synapse formation. Nature. 411:489–494. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

