Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis
- PMID: 11901200
- PMCID: PMC2193738
- DOI: 10.1084/jem.20011299
Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis
Abstract
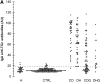
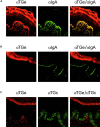
Gluten sensitivity typically presents as celiac disease, a common chronic small intestinal disorder. However, in certain individuals it is associated with dermatitis herpetiformis, a blistering skin disease characterized by granular IgA deposits in the papillary dermis. While tissue transglutaminase has been implicated as the major autoantigen of gluten sensitive disease, there has been no explanation as to why this condition appears in two distinct forms. Here we show that while sera from patients with either form of gluten sensitive disease react both with tissue transglutaminase and the related enzyme epidermal (type 3) transglutaminase, antibodies in patients having dermatitis herpetiformis show a markedly higher avidity for epidermal transglutaminase. Further, these patients have an antibody population specific for this enzyme. We also show that the IgA precipitates in the papillary dermis of patients with dermatitis herpetiformis, the defining signs of the disease, contain epidermal transglutaminase, but not tissue transglutaminase or keratinocyte transglutaminase. These findings demonstrate that epidermal transglutaminase, rather than tissue transglutaminase, is the dominant autoantigen in dermatitis herpetiformis and explain why skin symptoms appear in a proportion of patients having gluten sensitive disease.
Figures


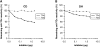
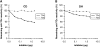


References
-
- Fry, L. 1995. Dermatitis herpetiformis. Baillière. Clin. Gastr. 9:371–394. - PubMed
-
- Sollid, L.M. 2000. Molecular basis of celiac disease. Annu. Rev. Immunol. 18:53–81. - PubMed
-
- Dieterich, W., T. Ehnis, M. Bauer, P. Donner, U. Volta, E.O. Riecken, and D. Schuppan. 1997. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat. Med. 3:797–801. - PubMed
-
- Dieterich, W., E. Laag, L. Bruckner-Tudermann, T. Reunala, S. Kárpáti, T. Zágoni, E.O. Riecken, and D. Schuppan. 1999. Antibodies to tissue transglutaminase as serologic markers in patients with dermatitis herpetiformis. J. Invest. Dermatol. 113:133–136. - PubMed
-
- Dieterich, W., E. Laag, H. Schöpper, U. Volta, A. Ferguson, H. Gillett, E.O. Riecken, and D. Schuppan. 1998. Autoantibodies to tissue transglutaminase as predictors of celiac disease. Gastroenterology. 115:1317–1321. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

