B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen
- PMID: 11901202
- PMCID: PMC2193734
- DOI: 10.1084/jem.20011140
B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen
Abstract
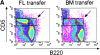
Splenectomized individuals are prone to overwhelming infections with encapsulated bacteria and splenectomy of mice increases susceptibility to streptococcal infections, yet the exact mechanism by which the spleen protects against such infections is unknown. Using congenitally asplenic mice as a model, we show that the spleen is essential for the generation of B-1a cells, a B cell population that cooperates with the innate immune system to control early bacterial and viral growth. Splenectomy of wild-type mice further demonstrated that the spleen is also important for the survival of B-1a cells. Transfer experiments demonstrate that lack of these cells, as opposed to the absence of the spleen per se, is associated with an inability to mount a rapid immune response against streptococcal polysaccharides. Thus, absence of the spleen and the associated increased susceptibility to streptococcal infections is correlated with lack of B-1a B cells. These findings reveal a hitherto unknown role of the spleen in generating and maintaining the B-1a B cell pool.
Figures







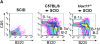
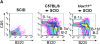







References
-
- Hansen, K., and D.B. Singer. 2001. Asplenic-hyposplenic overwhelming sepsis: postsplenectomy sepsis revisited. Ped. Dev. Pathol. 4:105–121. - PubMed
-
- Styrt, B. 1990. Infection associated with asplenia: risks, mechanisms, and prevention. Am. J. Med. 88:33N–42N. - PubMed
-
- Phoon, C.K., and C.A. Neill. 1994. Asplenia syndrome: insight into embryology through an analysis of cardiac and extracardiac anomalies. Am. J. Cardiol. 73:581–587. - PubMed
-
- Feder, H.M., Jr., and H.A. Pearson. 1999. Assessment of splenic function in familial asplenia. N. Engl. J. Med. 341:210–212. - PubMed
-
- Germing, U., C. Perings, S. Steiner, A.J. Peters, M.P. Heintzen, and C. Aul. 1999. Congenital asplenia detected in a 60 year old patient with septicemia. Eur. J. Med. Res. 28:283–285. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

