Pharmacological stimulation of the cholinergic antiinflammatory pathway
- PMID: 11901203
- PMCID: PMC2193742
- DOI: 10.1084/jem.20011714
Pharmacological stimulation of the cholinergic antiinflammatory pathway
Abstract
Efferent activity in the vagus nerve can prevent endotoxin-induced shock by attenuating tumor necrosis factor (TNF) synthesis. Termed the "cholinergic antiinflammatory pathway," inhibition of TNF synthesis is dependent on nicotinic alpha-bungarotoxin-sensitive acetylcholine receptors on macrophages. Vagus nerve firing is also stimulated by CNI-1493, a tetravalent guanylhydrazone molecule that inhibits systemic inflammation. Here, we studied the effects of pharmacological and electrical stimulation of the intact vagus nerve in adult male Lewis rats subjected to endotoxin-induced shock to determine whether intact vagus nerve signaling is required for the antiinflammatory action of CNI-1493. CNI-1493 administered via the intracerebroventricular route was 100,000-fold more effective in suppressing endotoxin-induced TNF release and shock as compared with intravenous dosing. Surgical or chemical vagotomy rendered animals sensitive to TNF release and shock, despite treatment with CNI-1493, indicating that an intact cholinergic antiinflammatory pathway is required for antiinflammatory efficacy in vivo. Electrical stimulation of either the right or left intact vagus nerve conferred significant protection against endotoxin-induced shock, and specifically attenuated serum and myocardial TNF, but not pulmonary TNF synthesis, as compared with sham-operated animals. Together, these results indicate that stimulation of the cholinergic antiinflammatory pathway by either pharmacological or electrical methods can attenuate the systemic inflammatory response to endotoxin-induced shock.
Figures
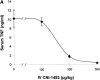










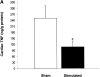

Comment in
-
Harnessing a neural-immune circuit to control inflammation and shock.J Exp Med. 2002 Mar 18;195(6):F25-8. doi: 10.1084/jem.20020602. J Exp Med. 2002. PMID: 11901206 Free PMC article. No abstract available.
References
-
- Tracey, K.J., B. Beutler, S.F. Lowry, J. Merryweather, S. Wolpe, I.W. Milsark, R.J. Hariri, T.J. Fahey, III, A. Zentalla, J.D. Albert, et al. 1986. Shock and tissue injury induced by recombinant human cachectin. Science. 234:470–474. - PubMed
-
- Wang, H., O. Bloom, M. Zhang, J.M. Vishnubhakat, M. Ombrellino, J. Che, A. Frazier, H. Yang, S. Ivanova, L. Borovikova, et al. 1999. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 285:248–251. - PubMed
-
- Borovikova, L.V., S. Ivanova, M. Zhang, H. Yang, G.I. Botchkina, L.R. Watkins, H. Wang, N. Abumrad, J.W. Eaton, and K.J. Tracey. 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 405:468–462. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

