Progress in the development of immunotherapy for the treatment of patients with cancer
- PMID: 11902815
- PMCID: PMC2413437
- DOI: 10.1046/j.1365-2796.2001.00911.x
Progress in the development of immunotherapy for the treatment of patients with cancer
Abstract
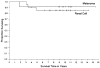
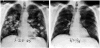
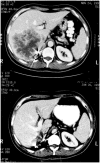
Several recent developments have hallmarked progress in tumour immunology and immunotherapy. The use of interleukin-2 (IL-2) in cancer patients demonstrated that an immunological manipulation was capable of mediating the regression of established growing cancers in humans. The identification of the genes encoding cancer antigens and the development of means for effectively immunizing patients against these antigens has opened important new avenues of exploration for the development of effective active and cell-transfer immunotherapies for patients with cancer.
Figures



References
-
- Morgan DA, Ruscetti FW, Gallo RG. Selective in vitro growth of T-lymphocytes from normal bone marrow. Science. 1986;193:1007. - PubMed
-
- Rosenberg SA, Schwarz S, Spiess PJ. In vitro growth of murine T-cells. II. Growth of in vitro sensitized cells cytotoxic for alloantigens. J Immunol. 1978;121:1951. - PubMed
-
- Strausser JL, Rosenberg SA. In vitro growth of cytotoxic human lymphocytes. I. Growth of cells sensitized in vitro to alloantigens. J Immunol. 1978;121:1491. - PubMed
-
- Lotze MT, Grimm EA, Mazumder A, Strausser JL, Rosenberg SA. Lysis of fresh and cultured autologous tumor by human lymphocytes cultured in T-cell growth factor (TCGF) Cancer Res. 1981;41:4420. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

