Mutation of the myosin converter domain alters cross-bridge elasticity
- PMID: 11904418
- PMCID: PMC122562
- DOI: 10.1073/pnas.062415899
Mutation of the myosin converter domain alters cross-bridge elasticity
Abstract
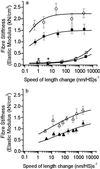
Elastic distortion of a structural element of the actomyosin complex is fundamental to the ability of myosin to generate motile forces. An elastic element allows strain to develop within the actomyosin complex (cross-bridge) before movement. Relief of this strain then drives filament sliding, or more generally, movement of a cargo. Even with the known crystal structure of the myosin head, however, the structural element of the actomyosin complex in which elastic distortion occurs remained unclear. To assign functional relevance to various structural elements of the myosin head, e.g., to identify the elastic element within the cross-bridge, we studied mechanical properties of muscle fibers from patients with familial hypertrophic cardiomyopathy with point mutations in the head domain of the beta-myosin heavy chain. We found that the Arg-719 --> Trp (Arg719Trp) mutation, which is located in the converter domain of the myosin head fragment, causes an increase in force generation and fiber stiffness under isometric conditions by 48-59%. Under rigor and relaxing conditions, fiber stiffness was 45-47% higher than in control fibers. Yet, kinetics of active cross-bridge cycling were unchanged. These findings, especially the increase in fiber stiffness under rigor conditions, indicate that cross-bridges with the Arg719Trp mutation are more resistant to elastic distortion. The data presented here strongly suggest that the converter domain that forms the junction between the catalytic and the light-chain-binding domain of the myosin head is not only essential for elastic distortion of the cross-bridge, but that the main elastic distortion may even occur within the converter domain itself.
Figures




References
-
- Rayment I, Holden H M, Whittaker M, Yohn C B, Lorenz M, Holmes K C, Milligan R A. Science. 1993;261:58–65. - PubMed
-
- Lowey S, Waller G S, Trybus K M. Nature (London) 1993;365:454–456. - PubMed
-
- Whittaker M, Wilson-Kubalek E M, Smith J E, Faust L, Milligan R A, Sweeney H L. Nature (London) 1995;378:748–751. - PubMed
-
- Dominguez R, Freyzon Y, Trybus K M, Cohen C. Cell. 1998;94:559–571. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

