Haploinsufficiency of mTR results in defects in telomere elongation
- PMID: 11904421
- PMCID: PMC122568
- DOI: 10.1073/pnas.012549799
Haploinsufficiency of mTR results in defects in telomere elongation
Abstract
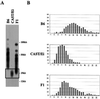
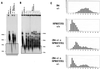
Telomeres are usually maintained about an equilibrium length, and the set point for this equilibrium differs between species and between strains of a given species. To examine the requirement for telomerase in mediating establishment of a new telomere length equilibrium, we generated interspecies crosses with telomerase mTR knockout mice. In crosses between C57BL/6J (B6) and either of two unrelated mouse species, CAST/Ei and SPRET/Ei, telomerase mediated establishment of a new telomere length equilibrium in wild-type mTR(+/+) mice. This new equilibrium was characterized by elongation of the short telomeres of CAST/Ei or SPRET/Ei origin. In contrast, mTR(-/-) offspring of interspecies crosses failed to elongate telomeres. Unexpectedly, haploinsufficiency was observed in mTR(+/-) heterozygous interspecies mice, which had an impaired ability to elongate short SPRET/Ei or CAST/Ei telomeres to the new equilibrium set point that was achieved in wild-type mTR(+/+) mice. These results demonstrate that elongation of telomeres to a new telomere set point requires telomerase and indicate that telomerase RNA may be limiting in vivo.
Figures



Similar articles
-
Telomere length is inherited with resetting of the telomere set-point.Proc Natl Acad Sci U S A. 2010 Jun 1;107(22):10148-53. doi: 10.1073/pnas.0913125107. Epub 2010 May 17. Proc Natl Acad Sci U S A. 2010. PMID: 20479226 Free PMC article.
-
Short telomeres, even in the presence of telomerase, limit tissue renewal capacity.Cell. 2005 Dec 16;123(6):1121-31. doi: 10.1016/j.cell.2005.11.020. Cell. 2005. PMID: 16360040
-
Short telomeres initiate telomere recombination in primary and tumor cells.PLoS Genet. 2009 Jan;5(1):e1000357. doi: 10.1371/journal.pgen.1000357. Epub 2009 Jan 30. PLoS Genet. 2009. PMID: 19180191 Free PMC article.
-
Telomerase RNA levels limit the telomere length equilibrium.Cold Spring Harb Symp Quant Biol. 2006;71:225-9. doi: 10.1101/sqb.2006.71.063. Cold Spring Harb Symp Quant Biol. 2006. PMID: 17381301 Review.
-
Telomerase caught in the act: united we stand, divided we fall.RNA Biol. 2012 Sep;9(9):1139-43. doi: 10.4161/rna.21498. Epub 2012 Sep 1. RNA Biol. 2012. PMID: 22951592 Free PMC article. Review.
Cited by
-
Premature ageing of lung alveoli and bone marrow cells from Terc deficient mice with different telomere lengths.Sci Rep. 2025 Feb 19;15(1):6102. doi: 10.1038/s41598-025-90246-2. Sci Rep. 2025. PMID: 39971959 Free PMC article.
-
Telomerase Mediates Lymphocyte Proliferation but Not the Atherosclerosis-Suppressive Potential of Regulatory T-Cells.Arterioscler Thromb Vasc Biol. 2018 Jun;38(6):1283-1296. doi: 10.1161/ATVBAHA.117.309940. Epub 2018 Mar 29. Arterioscler Thromb Vasc Biol. 2018. PMID: 29599138 Free PMC article.
-
Engineered telomere degradation models dyskeratosis congenita.Genes Dev. 2008 Jul 1;22(13):1773-85. doi: 10.1101/gad.1679208. Epub 2008 Jun 11. Genes Dev. 2008. PMID: 18550783 Free PMC article.
-
Function, replication and structure of the mammalian telomere.Cytotechnology. 2004 Jun;45(1-2):3-12. doi: 10.1007/s10616-004-5120-6. Cytotechnology. 2004. PMID: 19003238 Free PMC article.
-
Telomere length homeostasis requires that telomerase levels are limiting.EMBO J. 2006 Feb 8;25(3):565-74. doi: 10.1038/sj.emboj.7600952. Epub 2006 Jan 19. EMBO J. 2006. PMID: 16424902 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

