G protein-coupled receptor kinase 4 gene variants in human essential hypertension
- PMID: 11904438
- PMCID: PMC122616
- DOI: 10.1073/pnas.062694599
G protein-coupled receptor kinase 4 gene variants in human essential hypertension
Abstract
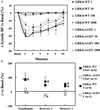
Essential hypertension has a heritability as high as 30-50%, but its genetic cause(s) has not been determined despite intensive investigation. The renal dopaminergic system exerts a pivotal role in maintaining fluid and electrolyte balance and participates in the pathogenesis of genetic hypertension. In genetic hypertension, the ability of dopamine and D(1)-like agonists to increase urinary sodium excretion is impaired. A defective coupling between the D(1) dopamine receptor and the G protein/effector enzyme complex in the proximal tubule of the kidney is the cause of the impaired renal dopaminergic action in genetic rodent and human essential hypertension. We now report that, in human essential hypertension, single nucleotide polymorphisms of a G protein-coupled receptor kinase, GRK4gamma, increase G protein-coupled receptor kinase (GRK) activity and cause the serine phosphorylation and uncoupling of the D(1) receptor from its G protein/effector enzyme complex in the renal proximal tubule and in transfected Chinese hamster ovary cells. Moreover, expressing GRK4gammaA142V but not the wild-type gene in transgenic mice produces hypertension and impairs the diuretic and natriuretic but not the hypotensive effects of D(1)-like agonist stimulation. These findings provide a mechanism for the D(1) receptor coupling defect in the kidney and may explain the inability of the kidney to properly excrete sodium in genetic hypertension.
Figures
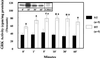



References
-
- Guyton A C. Circulatory Physiology III: Arterial Pressure and Hypertension. Philadelphia: Saunders; 1980.
-
- Guidi E, Menghetti D, Milani S, Montagnino G, Plazzi O, Bianchi G. J Am Soc Nephrol. 1996;7:1131–1138. - PubMed
-
- Jose P A, Eisner G M, Felder R A. Pharmacol Ther. 1998;80:149–182. - PubMed
-
- Hegde S S, Jadhav A L, Lokhandwala M F. Hypertension. 1989;13:828–834. - PubMed
-
- Felder R A, Seikaly M G, Cody P, Eisner G M, Jose P A. Hypertension. 1990;15:560–569. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

