Chronic intracellular infection of alfalfa nodules by Sinorhizobium meliloti requires correct lipopolysaccharide core
- PMID: 11904442
- PMCID: PMC122627
- DOI: 10.1073/pnas.062425699
Chronic intracellular infection of alfalfa nodules by Sinorhizobium meliloti requires correct lipopolysaccharide core
Abstract
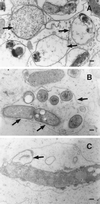
Our analyses of lipopolysaccharide mutants of Sinorhizobium meliloti offer insights into how this bacterium establishes the chronic intracellular infection of plant cells that is necessary for its nitrogen-fixing symbiosis with alfalfa. Derivatives of S. meliloti strain Rm1021 carrying an lpsB mutation are capable of colonizing curled root hairs and forming infection threads in alfalfa in a manner similar to a wild-type strain. However, developmental abnormalities occur in the bacterium and the plant at the stage when the bacteria invade the plant nodule cells. Loss-of-function lpsB mutations, which eliminate a protein of the glycosyltransferase I family, cause striking changes in the carbohydrate core of the lipopolysaccharide, including the absence of uronic acids and a 40-fold relative increase in xylose. We also found that lpsB mutants were sensitive to the cationic peptides melittin, polymyxin B, and poly-l-lysine, in a manner that paralleled that of Brucella abortus lipopolysaccharide mutants. Sensitivity to components of the plant's innate immune system may be part of the reason that this mutant is unable to properly sustain a chronic infection within the cells of its host-plant alfalfa.
Figures


References
-
- Brewin N J. Annu Rev Cell Biol. 1991;7:191–226. - PubMed
-
- Noel K D, Duelli D M. In: Prokaryotic Nitrogen Fixation: A Model System for Analysis of a Biological Process. Triplett E W, editor. Wymondham, U.K.: Horizon; 2000. pp. 415–431.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

