The vitelliform macular dystrophy protein defines a new family of chloride channels
- PMID: 11904445
- PMCID: PMC122639
- DOI: 10.1073/pnas.052692999
The vitelliform macular dystrophy protein defines a new family of chloride channels
Abstract
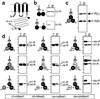
Vitelliform macular dystrophy (VMD/Best disease; MIM*153700) is an early-onset autosomal dominant disorder in which accumulation of lipofuscin-like material within and beneath the retinal pigment epithelium is associated with a progressive loss of central vision. Bestrophin, the protein product of the VMD gene, has four predicted transmembrane domains. There are multiple bestrophin homologues in the human, Drosophila, and Caenorhabditis elegans genomes, but no function has previously been ascribed to these proteins, and they show no detectable homology to other proteins of known function. Using heterologous expression, we show here that human, Drosophila, and C. elegans bestrophins form oligomeric chloride channels, and that human bestrophin is sensitive to intracellular calcium. Each of 15 missense mutations asscociated with VMD greatly reduces or abolishes the membrane current. Four of these mutant bestrophins were coexpressed with the wild type and each dominantly inhibited the wild-type membrane current, consistent with the dominant nature of the disease. These experiments establish the existence of a new chloride channel family and VMD as a channelopathy.
Figures
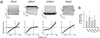
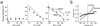
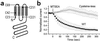

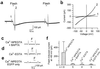

References
-
- Berger J W, Fine S L, Maguire M G. Age-Related Macular Degeneration. St. Louis: Mosby; 1999.
-
- Stone E M, Sheffield V C, Hageman G S. Hum Mol Genet. 2001;10:2285–2292. - PubMed
-
- Weingeist T A, Kobrin J L, Watzke R C. Arch Ophthalmol. 1982;100:1108–1114. - PubMed
-
- Frangieh G T, Green W R, Fine S L. Arch Ophthalmol. 1982;100:1115–1121. - PubMed
-
- Marquardt A, Stohr H, Passmore L A, Kramer F, Rivera A, Weber B H. Hum Mol Genet. 1998;7:1517–1525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

