Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection
- PMID: 11904450
- PMCID: PMC122648
- DOI: 10.1073/pnas.052712499
Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection
Abstract
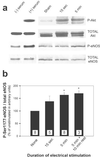
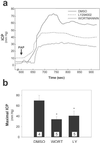
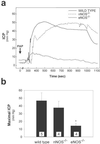
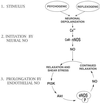
In the penis, nitric oxide (NO) can be formed by both neuronal NO synthase and endothelial NOS (eNOS). eNOS is activated by viscous drag/shear stress in blood vessels to produce NO continuously, a process mediated by the phosphatidylinositol 3-kinase (PI3kinase)/Akt pathway. Here we show that PI3-kinase/Akt physiologically mediates erection. Both electrical stimulation of the cavernous nerve and direct intracavernosal injection of the vasorelaxant drug papaverine cause rapid increases in phosphorylated (activated) Akt and eNOS. Phosphorylation is diminished by wortmannin and LY294002, inhibitors of PI3-kinase, the upstream activator of Akt. The two drugs also reduce erection. Penile erection elicited by papaverine is reduced profoundly in mice with targeted deletion of eNOS. Our findings support a model in which rapid, brief activation of neuronal NOS initiates the erectile process, whereas PI3-kinase/Akt-dependent phosphorylation and activation of eNOS leads to sustained NO production and maximal erection.
Figures


Similar articles
-
Nitric oxide mediates the antiapoptotic effect of insulin in myocardial ischemia-reperfusion: the roles of PI3-kinase, Akt, and endothelial nitric oxide synthase phosphorylation.Circulation. 2002 Mar 26;105(12):1497-502. doi: 10.1161/01.cir.0000012529.00367.0f. Circulation. 2002. PMID: 11914261
-
Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation.J Biol Chem. 2001 May 11;276(19):16587-91. doi: 10.1074/jbc.M100229200. Epub 2001 Feb 28. J Biol Chem. 2001. PMID: 11340086
-
Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells.Circ Res. 2000 Oct 13;87(8):677-82. doi: 10.1161/01.res.87.8.677. Circ Res. 2000. PMID: 11029403
-
Novel nitric oxide signaling mechanisms regulate the erectile response.Int J Impot Res. 2004 Jun;16 Suppl 1:S15-9. doi: 10.1038/sj.ijir.3901209. Int J Impot Res. 2004. PMID: 15224130 Review.
-
Posttranslational modification of constitutive nitric oxide synthase in the penis.J Androl. 2009 Jul-Aug;30(4):352-62. doi: 10.2164/jandrol.108.006999. Epub 2009 Apr 2. J Androl. 2009. PMID: 19342700 Free PMC article. Review.
Cited by
-
Sickling cells, cyclic nucleotides, and protein kinases: the pathophysiology of urogenital disorders in sickle cell anemia.Anemia. 2012;2012:723520. doi: 10.1155/2012/723520. Epub 2012 Jun 13. Anemia. 2012. PMID: 22745902 Free PMC article.
-
Improvement of erectile dysfunction using endothelial progenitor cells from fetal cerebral vasculature in the cavernous nerve injury of rats.Basic Clin Androl. 2022 Dec 1;32(1):21. doi: 10.1186/s12610-022-00171-x. Basic Clin Androl. 2022. PMID: 36451096 Free PMC article.
-
Preserved fertility despite erectile dysfunction in mice lacking the nitric oxide receptor.J Physiol. 2013 Jan 15;591(2):491-502. doi: 10.1113/jphysiol.2012.245555. Epub 2012 Nov 5. J Physiol. 2013. PMID: 23129789 Free PMC article.
-
Transnitrosylation: A Factor in Nitric Oxide-Mediated Penile Erection.J Sex Med. 2016 May;13(5):808-814. doi: 10.1016/j.jsxm.2016.03.003. J Sex Med. 2016. PMID: 27114194 Free PMC article.
-
Influence of arginase polymorphisms and arginase levels/activity on the response to erectile dysfunction therapy with sildenafil.Pharmacogenomics J. 2018 Apr;18(2):238-244. doi: 10.1038/tpj.2017.2. Epub 2017 Apr 4. Pharmacogenomics J. 2018. PMID: 28374859
References
-
- Bredt D S. Free Radical Res. 1999;31:577–596. - PubMed
-
- Bogdan C. Nat Immun. 2001;2:907–916. - PubMed
-
- Huang P L. J Am Soc Nephrol. 2000;11, Suppl. 16:S120–S123. - PubMed
-
- Stanarius A, Uckert S, Machtens S A, Stief C G, Wolf G, Jonas U. Urol Res. 2001;29:168–172. - PubMed
-
- Alm P, Larsson B, Ekblad E, Sundler F, Andersson K E. Acta Physiol Scand. 1993;148:421–429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

