AtKC1, a silent Arabidopsis potassium channel alpha -subunit modulates root hair K+ influx
- PMID: 11904452
- PMCID: PMC122651
- DOI: 10.1073/pnas.052677799
AtKC1, a silent Arabidopsis potassium channel alpha -subunit modulates root hair K+ influx
Abstract
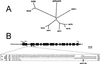
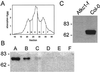
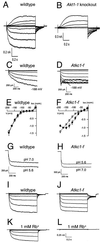
Ion channels in roots allow the plant to gain access to nutrients. The composition of the individual ion channels and the functional contribution of different alpha-subunits is largely unknown. Focusing on K(+)-selective ion channels, we have characterized AtKC1, a new alpha-subunit from the Arabidopsis shaker-like ion channel family. Promoter-beta-glucuronidase (GUS) studies identified AtKC1 expression predominantly in root hairs and root endodermis. Specific antibodies recognized AtKC1 at the plasma membrane. To analyze further the abundance and the functional contribution of the different K(+) channels alpha-subunits in root cells, we performed real-time reverse transcription-PCR and patch-clamp experiments on isolated root hair protoplasts. Studying all shaker-like ion channel alpha-subunits, we only found the K(+) inward rectifier AtKC1 and AKT1 and the K(+) outward rectifier GORK to be expressed in this cell type. Akt1 knockout plants essentially lacked inward rectifying K(+) currents. In contrast, inward rectifying K(+) currents were present in AtKC1 knockout plants, but fundamentally altered with respect to gating and cation sensitivity. This indicates that the AtKC1 alpha-subunit represents an integral component of functional root hair K(+) uptake channels.
Figures

References
-
- Gaymard F, Pilot G, Lacombe B, Bouchez D, Bruneau D, Boucherez J, Michaux-Ferriere N, Thibaud J B, Sentenac H. Cell. 1998;94:647–655. - PubMed
-
- Ache P, Becker D, Ivashikina N, Dietrich P, Roelfsema M R, Hedrich R. FEBS Lett. 2000;486:93–98. - PubMed
-
- Schachtman D P, Schroeder J I, Lucas W J, Anderson J A, Gaber R F. Science. 1994;258:1654–1658. - PubMed
-
- Bertl A, Anderson J A, Slayman C L, Sentenac H, Gaber R F. Folia Microbiol (Praha) 1994;39:507–509. - PubMed
-
- Pilot G, Lacombe B, Gaymard F, Cherel I, Boucherez J, Thibaud J B, Sentenac H. J Biol Chem. 2001;276:3215–3221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

