Sensitizing effects of lafutidine on CGRP-containing afferent nerves in the rat stomach
- PMID: 11906962
- PMCID: PMC1573261
- DOI: 10.1038/sj.bjp.0704596
Sensitizing effects of lafutidine on CGRP-containing afferent nerves in the rat stomach
Abstract
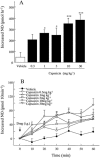
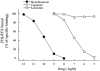
1. Capsaicin sensitive afferent nerves play an important role in gastric mucosal defensive mechanisms. Capsaicin stimulates afferent nerves and enhances the release of calcitonin gene-related peptide (CGRP), which seems to be the predominant neurotransmitter of spinal afferents in the rat stomach, exerting many pharmacological effects by a direct mechanism or indirectly through second messengers such as nitric oxide (NO). 2. Lafutidine is a new type of anti-ulcer drug, possessing both an antisecretory effect, exerted via histamine H(2) receptor blockade, and gastroprotective activities. Studies with certain antagonists or chemical deafferentation techniques suggest the gastroprotective actions of lafutidine to be mediated by capsaicin sensitive afferent nerves, but this is an assumption based on indirect techniques. In order to explain the direct relation of lafutidine to afferent nerves, we conducted the following studies. 3. We determined CGRP and NO release from rat stomach and specific [(3)H]-resiniferatoxin (RTX) binding to gastric vanilloid receptor subtype 1 (VR1), which binds capsaicin, using EIA, a microdialysis system and a radioreceptor assay, respectively. 4. Lafutidine enhanced both CGRP and NO release from the rat stomach induced by a submaximal dose of capsaicin, but had no effect on specific [(3)H]-RTX and capsaicin binding to VR1. 5. In conclusion, our findings demonstrate that lafutidine modulates the activity of capsaicin sensitive afferent nerves in the rat stomach, which may be a key mechanism involved in its gastroprotective action.
Figures





References
-
- ABDEL-SALAM O.M.E., DEBRECENI A., MOZSIK G., SZOLCSANYI J. Capsaicin-sensitive afferent sensory nerves in modulating gastric mucosal defense against noxious agents. J. Physiol. Paris. 1999;93:443–454. - PubMed
-
- AJIOKA H., MIYAKE H., MATSUURA N. Effect of FRG-8813, a new-type histamine H2-receptor antagonist, on the recurrence of gastric ulcer after healing by drug treatment in rats. Pharmacology. 2000;61:83–90. - PubMed
-
- BROWN J.F., HANSON P.J., WHITTLE B.J.R. Nitric oxide donors increase mucus gel thickness in rat stomach. Eur. J. Pharmacol. 1992a;223:103–104. - PubMed
-
- BROWN J.F., KEATES A.C., HANSON P.J., WHITTLE B.J.R. Nitric oxide generators and cGMP stimulate mucus secretion by rat gastric mucosal cells. Am. J. Physiol. 1993;265:G418–G422. - PubMed
-
- BROWN J.F., TEPPERMAN B.L., HANSON P.J., WHITTLE B.J.R., MONCADA S. Differential distribution of nitric oxide synthase between cell fractions isolated from the rat gastric mucosa. Biochem. Biophys. Res. Commun. 1992b;184:680–685. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

