Kunjin virus replicon vaccine vectors induce protective CD8+ T-cell immunity
- PMID: 11907219
- PMCID: PMC136104
- DOI: 10.1128/jvi.76.8.3791-3799.2002
Kunjin virus replicon vaccine vectors induce protective CD8+ T-cell immunity
Abstract
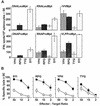
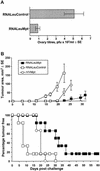
The ability of self-replicating RNA (replicon) vaccine vectors derived from the Australian flavivirus Kunjin (KUN) to induce protective alphabeta CD8+ T-cell responses was examined. KUN replicons encoding a model immunogen were delivered by three different vaccine modalities: (i) as naked RNA transcribed in vitro, (ii) as plasmid DNA constructed to allow in vivo transcription of replicon RNA by cellular RNA polymerase II (DNA based), and (iii) as replicon RNA encapsidated into virus-like particles. A single immunization with any of these KUN replicon vaccines induced CD8+ T-cell responses at levels comparable to those induced by recombinant vaccinia virus encoding the same immunogen. Immunization with only 0.1 microg of DNA-based KUN replicons elicited CD8+ T-cell responses similar to those seen after immunization with 100 microg of a conventional DNA vaccine. Naked RNA immunization with KUN replicons also protected mice against challenges with recombinant vaccinia virus and B16 tumor cells. These results demonstrate the value of KUN replicon vectors for inducing protective antiviral and anticancer CD8+ T-cell responses.
Figures



References
-
- Albert, M. L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392:86-89. - PubMed
-
- Bellone, M., D. Cantarella, P. Castiglioni, M. C. Crosti, A. Ronchetti, M. Moro, M. P. Garancini, G. Casorati, and P. Dellabona. 2000. Relevance of the tumor antigen in the validation of three vaccination strategies for melanoma. J. Immunol. 165:2651-2656. - PubMed
-
- Berglund, P., C. Smerdou, M. N. Fleeton, I. Tubulekas, and P. Liljestrom. 1998. Enhancing immune responses using suicidal DNA vaccines. Nat. Biotechnol. 16:562-565. - PubMed
-
- Binder, R. J., D. K. Han, and P. K. Srivastava. 2000. CD91: a receptor for heat shock protein gp96. Nat. Immunol. 1:151-155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

