Preferential localization of the Epstein-Barr virus (EBV) oncoprotein LMP-1 to nuclei in human T cells: implications for its role in the development of EBV genome-positive T-cell lymphomas
- PMID: 11907247
- PMCID: PMC136072
- DOI: 10.1128/jvi.76.8.4080-4086.2002
Preferential localization of the Epstein-Barr virus (EBV) oncoprotein LMP-1 to nuclei in human T cells: implications for its role in the development of EBV genome-positive T-cell lymphomas
Abstract
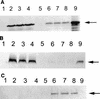
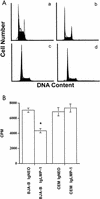
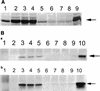
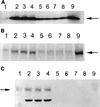
The Epstein-Barr virus (EBV)-encoded latent membrane protein-1 (LMP-1) is thought to play a role in the EBV-induced B-cell transformation and immortalization. EBV has also been implicated in certain human T-cell lymphomas; however, the phenotypic effects of the expression of this oncoprotein in T cells are not known. To learn whether LMP-1 also induces phenotypic changes in T cells, we stably expressed it in human cell lines of T and B lineages and 25 LMP-1-expressing T-cell clones and 7 B-cell clones were examined. Our results show for the first time that, in sharp contrast to B cells, LMP-1 preferentially localizes to nuclei in T cells and does not induce the phenotypic changes in these cells that it induces in B cells, does not associate with TRAF proteins, and does not arrest the cell cycle in the G2/M phase. A computer-assisted analysis revealed that LMP-1 lacks the canonical nuclear localization signal. Our results suggest that this oncoprotein may not play the same role in the lymphomagenesis of T cells as it does in B cells.
Figures


References
-
- Ahmad, A., A. Ladha, E. A. Cohen, and J. Menezes. 1993. Stable expression of the transfected HIV-1 env gene in a human B cell line: characterization of gp120-expressing clones and immunobiological studies. Virology 192:447-457. - PubMed
-
- Arvanitakis, L., N. Yaseen, and S. Sharma. 1995. Latent membrane protein-1 induces cyclin D2 expression, pRb hyperphosphorylation, and loss of TGF-beta 1-mediated growth inhibition in EBV-positive B cells. J. Immunol. 155:1047-1056. - PubMed
-
- Babcock, G. J., L. L. Decker, M. Volk, and D. A. Thorley-Lawson. 1998. EBV persistence in memory B cells in vivo. Immunity 9:395-404. - PubMed
-
- Bonagura, V. R., B. Z. Katz, B. C. Edwards, D. J. Valaces, P. Nisen, E. Gloster, R. Mir, and P. Lanzkowsky. 1990. Severe chronic EBV infection associated with specific EBV immunodeficiency and an EBNA+ T-cell lymphoma containing linear, EBV DNA. Clin. Immunol. Immunopathol. 57:32-44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

