Relation between burden of disease and randomised evidence in sub-Saharan Africa: survey of research
- PMID: 11909786
- PMCID: PMC99053
- DOI: 10.1136/bmj.324.7339.702
Relation between burden of disease and randomised evidence in sub-Saharan Africa: survey of research
Abstract
Objective: To evaluate whether the amount of randomised clinical research on various medical conditions is related to the burden of disease and health needs of the local populations in sub-Saharan Africa.
Design: Construction and analysis of comprehensive database of randomised controlled trials in sub-Saharan Africa based on Medline, the Cochrane Controlled Trials Register, and several African databases.
Setting: Sub-Saharan Africa.
Main outcome measures: Number of trials and randomised subjects for each category of disease in the global burden of disease taxonomy; ratios of disability adjusted life years (DALYs) per amount of randomised evidence.
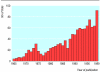
Results: 1179 eligible randomised controlled trials were identified. The number of trials published each year increased over time. Almost half of the trials (n=565) had been done in South Africa. There was relatively good correlation between the estimated burden of disease at year 2000 and the number of trials performed (r=0.53, P=0.024) and the number of participants randomised (r=0.68, P=0.002). However,some conditions-for example, injuries (over 20 000 DALYs per patient ever randomised)-were more neglected than others.
Conclusion: Despite recent improvements, few clinical trials are done in sub-Saharan Africa. Clinical research in this part of the world should focus more evenly on the major contributors to burden of disease.
Figures


Comment in
-
Africa can solve its own health problems.BMJ. 2002 Mar 23;324(7339):688-9. doi: 10.1136/bmj.324.7339.688. BMJ. 2002. PMID: 11909770 Free PMC article. No abstract available.
References
-
- Gross CP, Anderson GF, Powe NR. The relation between funding by the National Institutes of Health and the burden of disease. N Engl J Med. 1999;340:1881–1887. - PubMed
-
- Murray CJL, Lopez AD. Global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Boston, MA: Harvard School of Public Health, World Health Organization, World Bank; 1996.
-
- Horton R. North and South: bridging the information gap. Lancet. 2000;355:2231–2236. - PubMed
-
- United Nations Statistical Division. Monthly bulletin of statistics. New York: UN; 1992. ;XLVI:No 7.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
