SRC catalytic but not scaffolding function is needed for integrin-regulated tyrosine phosphorylation, cell migration, and cell spreading
- PMID: 11909938
- PMCID: PMC133722
- DOI: 10.1128/MCB.22.8.2427-2440.2002
SRC catalytic but not scaffolding function is needed for integrin-regulated tyrosine phosphorylation, cell migration, and cell spreading
Abstract
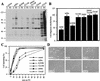
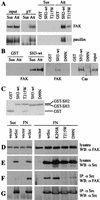
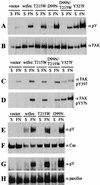
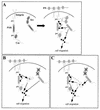
Src family kinases (SFKs) are crucial for signaling through a variety of cell surface receptors, including integrins. There is evidence that integrin activation induces focal adhesion kinase (FAK) autophosphorylation at Y397 and that Src binds to and is activated by FAK to carry out subsequent phosphorylation events. However, it has also been suggested that Src functions as a scaffolding molecule through its SH2 and SH3 domains and that its kinase activity is not necessary. To examine the role of SFKs in integrin signaling, we have expressed various Src molecules in fibroblasts lacking other SFKs. In cells plated on fibronectin, FAK could indeed autophosphorylate at Y397 independently of Src but with lower efficiency than when Src was present. This step was promoted by kinase-inactive Src, but Src kinase activity was required for full rescue. Src kinase activity was also required for phosphorylation of additional sites on FAK and for other integrin-directed functions, including cell migration and spreading on fibronectin. In contrast, Src mutations in the SH2 or SH3 domain greatly reduced binding to FAK, Cas, and paxillin but had little effect on tyrosine phosphorylation or biological assays. Furthermore, our indirect evidence indicates that Src kinase activity does not need to be regulated to promote cell migration and FAK phosphorylation. Although Src clearly plays important roles in integrin signaling, it was not concentrated in focal adhesions. These results indicate that the primary role of Src in integrin signaling is as a kinase. Indirect models for Src function are proposed.
Figures

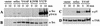


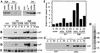
References
-
- Angers-Loustau, A., J. F. Cote, and M. L. Tremblay. 1999. Roles of protein tyrosine phosphatases in cell migration and adhesion. Biochem. Cell Biol. 77:493-505. - PubMed
-
- Avraham, H., S. Y. Park, K. Schinkmann, and S. Avraham. 2000. RAFTK/Pyk2-mediated cellular signalling. Cell. Signal. 12:123-133. - PubMed
-
- Boerner, R. J., D. B. Kassel, S. C. Barker, B. Ellis, P. DeLacy, and W. B. Knight. 1996. Correlation of the phosphorylation states of pp60c-src with tyrosine kinase activity: the intramolecular pY530-SH2 complex retains significant activity if Y419 is phosphorylated. Biochemistry 35:9519-9525. - PubMed
-
- Brown, M. T., and J. A. Cooper. 1996. Regulation, substrates and functions of src. Biochim. Biophys. Acta 1287:121-149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
