The response of c-jun/AP-1 to chronic hypoxia is hypoxia-inducible factor 1 alpha dependent
- PMID: 11909946
- PMCID: PMC133718
- DOI: 10.1128/MCB.22.8.2515-2523.2002
The response of c-jun/AP-1 to chronic hypoxia is hypoxia-inducible factor 1 alpha dependent
Abstract
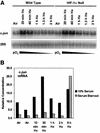
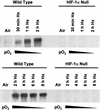
Hypoxia (low-oxygen tension) is an important physiological stress that influences responses to a wide range of pathologies, including stroke, infarction, and tumorigenesis. Prolonged or chronic hypoxia stimulates expression of the stress-inducible transcription factor gene c-jun and transient activation of protein kinase and phosphatase activities that regulate c-Jun/AP-1 activity. Here we describe evidence obtained by using wild-type and HIF-1 alpha nullizygous mouse embryonic fibroblasts (mEFs) that the induction of c-jun mRNA expression and c-Jun phosphorylation by prolonged hypoxia are completely dependent on the presence of the oxygen-regulated transcription factor hypoxia-inducible factor 1 alpha (HIF-1 alpha). In contrast, transient hypoxia induced c-jun expression in both types of mEFs, showing that the early or rapid induction of this gene is independent of HIF-1 alpha. These findings indicate that the c-jun gene has a biphasic response to hypoxia consisting of inductions that depend on the degree or duration of exposure. To more completely define the relationship between prolonged hypoxia and c-Jun phosphorylation, we used mEFs from mice containing inactivating mutations of critical phosphorylation sites in the c-Jun N-terminal region (serines 63 and 73 or threonines 91 and 93). Exposure of these mEFs to prolonged hypoxia demonstrated an absolute requirement for N-terminal sites for HIF-1 alpha-dependent phosphorylation of c-Jun. Taken together, these findings suggest that c-Jun/AP-1 and HIF-1 cooperate to regulate gene expression in pathophysiological microenvironments.
Figures

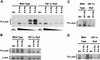
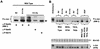

References
-
- Angel, P., K. Hattori, T. Smeal, and M. Karin. 1988. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell 55:875-885. - PubMed
-
- Angel, P., and M. Karin. 1991. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta 1072:129-157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
